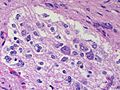
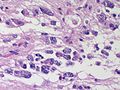
Difference between revisions of "Neuropathology tumours"
Jensflorian (talk | contribs) |
Jensflorian (talk | contribs) (Minor rearrangement) |
||
| (37 intermediate revisions by 2 users not shown) | |||
| Line 16: | Line 16: | ||
#*[[Melanoma]]. | #*[[Melanoma]]. | ||
#*[[Renal cell carcinoma]] (RCC). | #*[[Renal cell carcinoma]] (RCC). | ||
# [[Glioblastoma]] | # [[Glioblastoma]], IDH-wildtype. | ||
# [[ | # [[Astrocytoma, IDH-mutant]]. | ||
# [[Meningioma]]. | # [[Meningioma]]. | ||
| Line 24: | Line 24: | ||
# [[Medulloblastoma]]. | # [[Medulloblastoma]]. | ||
# [[Ependymoma]]. | # [[Ependymoma]]. | ||
# Pontine glioma, often [[Diffuse midline glioma, H3 K27-altered]]. | |||
===By location=== | ===By location=== | ||
| Line 92: | Line 93: | ||
* [[Olfactory neuroblastoma]]. | * [[Olfactory neuroblastoma]]. | ||
* [[Endolymphatic sac tumour]]. | * [[Endolymphatic sac tumour]]. | ||
===Primary versus secondary=== | ===Primary versus secondary=== | ||
*[[AKA]] (primary) brain tumour versus metastatic cancer. | *[[AKA]] (primary) brain tumour versus metastatic cancer. | ||
====Primary==== | ====Primary==== | ||
Glial tumours: | [[Glioma|Glial tumours]]: | ||
*Cytoplasmic processes - '''key feature'''. | *Cytoplasmic processes - '''key feature'''. | ||
**Best seen at highest magnification - usu. ~1 micrometer. | **Best seen at highest magnification - usu. ~1 micrometer. | ||
**Processes may branch. | **Processes may branch. | ||
*Ill-defined border/blend with the surrounding brain. | *Ill-defined border/blend with the surrounding brain. | ||
[[Meningioma]]: | |||
*Lesion often dura-based. | |||
*Mesenchymal tumor (often contains collagen). | |||
[[Lymphoma]]: | [[Lymphoma]]: | ||
*Primary CNS Lymphoma (PCNSL) is usu. a diffuse large B-cell lymphoma. | |||
*Large (lymphoid) cells, ergo usu. not a difficult diagnosis. | *Large (lymphoid) cells, ergo usu. not a difficult diagnosis. | ||
**~2x size of resting lymphocyte, nucleoli. | **~2x size of resting lymphocyte, nucleoli. | ||
*Lesion predominantly perivascular. | *Lesion predominantly perivascular. | ||
====Secondary==== | ====Secondary==== | ||
Carcinomas: | *Carcinomas: | ||
*Well-demarcated border between brain and lesion - '''key feature'''. | **Well-demarcated border between brain and lesion - '''key feature'''. | ||
*No cytoplasmic processes. | **No cytoplasmic processes. | ||
*Usu. have nuclear atypia of malignancy. | **Usu. have nuclear atypia of malignancy. | ||
**Nuclei often ~3-4x the size of a [[RBC]]. | **Nuclei often ~3-4x the size of a [[RBC]]. | ||
*+/-Glandular arrangement. | **+/-Glandular arrangement. | ||
*+/-Nucleoli. | **+/-Nucleoli. | ||
*Melanoma. | |||
*Secondary Lymphoma. | |||
*Sarcomas (rare). | |||
===By growth pattern=== | |||
====Infiltrative astrocytomas==== | |||
*[[Astrocytoma, IDH-mutant]]. | |||
*[[Glioblastoma]], IDH-wildtype. | |||
Notes: | |||
**Glial: "blends into brain"/gradual transition to non-tumour brain. | |||
====Non-infiltrative astrocytomas==== | |||
**[[Pilocytic astrocytoma]] | |||
**[[Pleomorphic xanthoastrocytoma]] | |||
**[[Subependymal giant cell astrocytoma]]. | |||
====Cystic tumours==== | |||
DDx:<ref>URL: [http://path.upmc.edu/cases/case320/dx.html http://path.upmc.edu/cases/case320/dx.html]. Accessed on: 14 January 2012.</ref> | |||
*[[Pilocytic astrocytoma]]. | |||
*[[Pleomorphic xanthoastrocytoma]]. | |||
*[[Ganglioglioma]]. | |||
*[[Hemangioblastoma]]. | |||
*[[Craniopharyngioma]].<ref>URL: [http://www.pathologyoutlines.com/Cnstumor.html#cystsgeneral http://www.pathologyoutlines.com/Cnstumor.html#cystsgeneral]. Accessed on: 14 January 2012.</ref> | |||
Notes: | |||
**Non-glial: no radiating glial processes. | |||
*Rosenthal fibres within the tumour... often seen in [[pilocytic astrocytoma]]. | |||
**Rosenthal fibres may be seen around a (very) slow growing tumour and represent a reactive process. | |||
*Inflammatory cells and macrophages should prompt consideration of an alternate diagnosis (e.g. [[cerebral infarct]], [[multiple sclerosis]]) - esp. if this is a primary lesion.<ref>URL: [http://path.upmc.edu/cases/case79/dx.html http://path.upmc.edu/cases/case79/dx.html]. Accessed on: 2 January 2012.</ref> | |||
====Grading==== | |||
Nuclear pleomorphism present: | |||
*At least grade II (diffuse astrocytoma). | |||
Mitotic figures present: | |||
*At least grade III (anaplastic astrocytoma). | |||
Microvascular proliferation ''or'' necrosis with pseudopalisading tumour cells: | |||
*Grade IV (glioblastoma [[AKA]] glioblastoma multiforme). | |||
Notes: | |||
*Pseudopalisading tumour cells = high tumour cell density adjacent to regions of necrosis; palisade = a fence of poles forming a defensive barrier or fortification. | |||
*WHO Grading is currently based on expected biologiocal behaviour without treatment. | |||
**Grading does not reflect molecular divergent groups within a tumor class or response to therapy (Currently controversies in grading for IDH-mutant astrocytoma vs. IDH-wildtype astrocytoma).<ref>{{Cite journal | last1 = Louis | first1 = DN. | last2 = von Deimling | first2 = A. | title = Grading of diffuse astrocytic gliomas: Broders, Kernohan, Zülch, the WHO… and Shakespeare. | journal = Acta Neuropathol | volume = | issue = | pages = | month = Aug | year = 2017 | doi = 10.1007/s00401-017-1765-z | PMID = 28801693 }}</ref> | |||
===By IHC=== | |||
*GFAP - should stain cytoplasm of tumour cells and the perikaryon (nuclear membrane) of most [[Astrocytoma]]s. | |||
*[[IDH-1]](R132H) (isocitrate dehydrogenase 1) in [[Astrocytoma, IDH-mutant]].<ref name=pmid19228619>{{cite journal |author=Yan H, Parsons DW, Jin G, ''et al.'' |title=IDH1 and IDH2 mutations in gliomas |journal=N. Engl. J. Med. |volume=360 |issue=8 |pages=765–73 |year=2009 |month=February |pmid=19228619 |pmc=2820383 |doi=10.1056/NEJMoa0808710 |url=}}</ref><ref name=pmid20975057>{{cite journal |author=Houillier C, Wang X, Kaloshi G, ''et al.'' |title=IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas |journal=Neurology |volume=75 |issue=17 |pages=1560–6 |year=2010 |month=October |pmid=20975057 |doi=10.1212/WNL.0b013e3181f96282 |url=}}</ref> | |||
*[[H3F3A|H3F3A K27M]] in [[Diffuse midline glioma, H3 K27-altered]]. | |||
*[[ATRX]] -ve in [[Astrocytoma, IDH-mutant]] or [[Diffuse hemispheric glioma, H3 G34-mutant]]. | |||
*[[CD20]] in PCNSL. | |||
*Cytokeratins in Carcinoma brain metastases, Plexus choroid tumours, [[AT/RT]], [[Papillary tumour of the pineal region]], [[Craniopharyngioma]]. | |||
*[[EMA]] in [[Meningioma]] and carcinoma brain metastases. | |||
*PrgR in [[Meningioma]] and carcinoma metastases. | |||
*[[Synaptophysin]] in glioneuronal tumours and Pituitary adenoma and embryonal tumours. | |||
===Common neuropathology tumours in a table=== | ===Common neuropathology tumours in a table=== | ||
| Line 147: | Line 202: | ||
|variable | |variable | ||
|missed lesion / close to a lesion; non-specific pathologic process - need more tissue | |missed lesion / close to a lesion; non-specific pathologic process - need more tissue | ||
| | |GFAP | ||
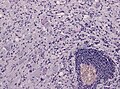
|[[Image:Reactive_astrocytes_-_lfb_-_high_mag.jpg|thumb|center|150px|Reactive astrocytes. (WC)]] | |[[Image:Reactive_astrocytes_-_lfb_-_high_mag.jpg|thumb|center|150px|Reactive astrocytes. (WC)]] | ||
|- | |- | ||
| Line 155: | Line 210: | ||
|old or young | |old or young | ||
|need frozen section to Dx, DDx: [[meningioma]] | |need frozen section to Dx, DDx: [[meningioma]] | ||
|S100 | |S100, SOX10 | ||
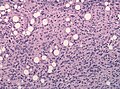
|[[Image:Schwannoma_-_Antoni_A_and_B_-_very_high_mag.jpg|thumb|center|150px|Schwannoma. (WC)]] | |[[Image:Schwannoma_-_Antoni_A_and_B_-_very_high_mag.jpg|thumb|center|150px|Schwannoma. (WC)]] | ||
|- | |- | ||
| Line 166: | Line 221: | ||
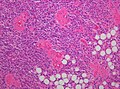
|[[Image:Meningioma_intermed_mag.jpg |thumb|center|150px|Meningioma. (WC)]] | |[[Image:Meningioma_intermed_mag.jpg |thumb|center|150px|Meningioma. (WC)]] | ||
|- | |- | ||
| | |[[Astrocytoma, IDH-mutant]] (CNS [[WHO]] grade 2 or grade 3) | ||
|glial processes (esp. on smear), nuclear atypia (typical size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion †, no microvascular proliferation, no necrosis | |glial processes (esp. on smear), nuclear atypia (typical size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion †, no microvascular proliferation, no necrosis | ||
|often enhancing (suggests high grade), usu. supratentorial, usu. white matter | |often enhancing (suggests high grade), usu. supratentorial, usu. white matter | ||
|usu. old, occ. young | |usu. old, occ. young | ||
|common | |common | ||
|IDH-1+/-, GFAP+ | |IDH-1(R132H)+/-, GFAP+ | ||
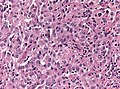
| [[Image:Anaplastic_astrocytoma_-_very_high_mag_-_cropped.jpg | thumb| center| 150px|High-grade astrocytoma. (WC)]] | | [[Image:Anaplastic_astrocytoma_-_very_high_mag_-_cropped.jpg | thumb| center| 150px|High-grade astrocytoma. (WC)]] | ||
|- | |- | ||
|[[Glioblastoma]] (WHO grade | |[[Glioblastoma]], IDH-wildtype (CNS [[WHO]] grade 4) | ||
|glial processes (esp. on smear), nuclear atypia (typical size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion †, microvascular proliferation or necrosis | |glial processes (esp. on smear), nuclear atypia (typical size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion †, microvascular proliferation or necrosis | ||
|often enhancing (suggests high grade), usu. supratentorial, usu. white matter | |often enhancing (suggests high grade), usu. supratentorial, usu. white matter | ||
| Line 195: | Line 250: | ||
{{Main|Brain metastasis}} | {{Main|Brain metastasis}} | ||
== | |||
===Molecular=== | |||
See also: [[Molecular_pathology_tests#Neuropathology|Molecular Neuropathology]] | |||
==Gliomas== | |||
{{Main|Glioma}} | |||
Gliomas, glioneuronal tumours and neuronal tumours are often categorized together. | |||
===Astrocytic tumours=== | |||
{{Main|Astrocytoma}} | {{Main|Astrocytoma}} | ||
* [[Astrocytoma]], IDH-mutant. | |||
* [[Glioblastoma]], IDH-wildtype. | |||
** [[Gliosarcoma]] (a glioblastoma subtype) | |||
*[[ | |||
Features:<ref name=pmid>{{cite journal |author=Rong Y, Durden DL, Van Meir EG, Brat DJ |title='Pseudopalisading' necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis |journal=J. Neuropathol. Exp. Neurol. |volume=65 |issue=6 |pages=529–39 |year=2006 |month=June |pmid=16783163 |doi= |url=}}</ref><ref>[http://dictionary.reference.com/browse/palisading http://dictionary.reference.com/browse/palisading]</ref> | Features:<ref name=pmid>{{cite journal |author=Rong Y, Durden DL, Van Meir EG, Brat DJ |title='Pseudopalisading' necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis |journal=J. Neuropathol. Exp. Neurol. |volume=65 |issue=6 |pages=529–39 |year=2006 |month=June |pmid=16783163 |doi= |url=}}</ref><ref>[http://dictionary.reference.com/browse/palisading http://dictionary.reference.com/browse/palisading]</ref> | ||
*Glial processes - '''key feature'''. | *Glial processes - '''key feature'''. | ||
| Line 223: | Line 276: | ||
*[http://path.upmc.edu/cases/case368.html Gemistocytic astrocytoma - several images (upmc.edu)]. | *[http://path.upmc.edu/cases/case368.html Gemistocytic astrocytoma - several images (upmc.edu)]. | ||
Depreceated: | |||
* | * Diffuse [[Astrocytoma]] | ||
* | * [[Anaplastic astrocytoma]] | ||
* | * [[Gliomatosis cerebri]] | ||
* Spongioblastoma | |||
* | |||
* | ===Oligodendroglial tumours=== | ||
* [[Oligodendroglioma]], IDH-mutant and 1p/19q codeleted. | |||
Depreceated: | |||
* Anaplastic oligodendroglioma | |||
* | * [[Oligoastrocytoma]] | ||
* Anaplastic oligoastrocytoma | |||
===Pediatric-type diffuse high-grade glioma=== | |||
* | {{Main|Pediatric-type diffuse high-grade glioma}} | ||
* [[Astrocytoma#Diffuse_midline_glioma.2C_H3_K27M_mutant|Diffuse midline glioma H3 K27-mutant]] | |||
===Pediatric-type diffuse low-grade glioma=== | |||
{{Main|Pediatric-type diffuse low-grade glioma}} | |||
=== | ===Circumscribed astrocytic gliomas=== | ||
* [[Pilocytic astrocytoma]] (PA) | |||
** [[Pilomyxoid astrocytoma]] (PMA) | |||
* [[Pleomorphic xanthoastrocytoma]] (PXA) | |||
* [[Subependymal giant cell astrocytoma]] (SEGA) | |||
* [[Neuropathology_tumours#Astroblastoma|Astroblastoma MN1-altered]]. | |||
* [[Neuropathology_tumours#Chordoid glioma of the third ventricl|Chordoid glioma]]. | |||
==== | ====Astroblastoma==== | ||
*No WHO grade yet.<ref>{{Ref WHOCNS|88}}</ref> | |||
{| | *Very rare superficial tumor of young age.<ref>{{Cite journal | last1 = Narayan | first1 = S. | last2 = Kapoor | first2 = A. | last3 = Singhal | first3 = MK. | last4 = Jakhar | first4 = SL. | last5 = Bagri | first5 = PK. | last6 = Rajput | first6 = PS. | last7 = Kumar | first7 = HS. | title = Astroblastoma of cerebrum: A rare case report and review of literature. | journal = J Cancer Res Ther | volume = 11 | issue = 3 | pages = 667 | month = | year = | doi = 10.4103/0973-1482.140800 | PMID = 26458709 }}</ref> | ||
*Large, cystic. Pushing margin towards CNS. | |||
| | *Vasocentric growth, plump cells with absence of fibrillary pattern. | ||
| | *GFAP+ve, Synaptohysin-ve, Olig-2-ve, focally EMA/panCK+ve. MIB-1: 1-18 %. | ||
*Molecular profile overlaps with classical [[CNS-PNET]]. | |||
**Gene fusions invoving meningioma gene (MN1)<ref>{{Cite journal | last1 = Sturm | first1 = D. | last2 = Orr | first2 = BA. | last3 = Toprak | first3 = UH. | last4 = Hovestadt | first4 = V. | last5 = Jones | first5 = DT. | last6 = Capper | first6 = D. | last7 = Sill | first7 = M. | last8 = Buchhalter | first8 = I. | last9 = Northcott | first9 = PA. | title = New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. | journal = Cell | volume = 164 | issue = 5 | pages = 1060-72 | month = Feb | year = 2016 | doi = 10.1016/j.cell.2016.01.015 | PMID = 26919435 }}</ref> | |||
| | |||
| | |||
| | |||
<gallery> | |||
File:Astroblastoma_HE_Specimen.jpg | HE. (WC/jensflorian) | |||
File:Astroblastoma_HE_papillae.jpg | HE. (WC/jensflorian) | |||
File:Astroblastoma.jpg | Astroblastoma (AFIP) | |||
</gallery> | |||
== | ====Chordoid glioma of the third ventricle==== | ||
{{ | * WHO grade II. | ||
* Slowly growing, non-invasive, in adults. | |||
* Clusters of epithelioid cells in mucinous stroma. | |||
* Lymphocytic infiltrates, adjacent Rosenthal fibers. | |||
* Fibrosis may be present. | |||
* Few mitoses. | |||
* [[GFAP]]+ve, MIB-1 1-3%. | |||
* [[TTF-1]]+ve. | |||
* CD34+ve. | |||
* [[IDH-1]]-ve, [[p53]]-ve. | |||
* PRKCA D463H mutations.<ref>{{Cite journal | last1 = Goode | first1 = B. | last2 = Mondal | first2 = G. | last3 = Hyun | first3 = M. | last4 = Ruiz | first4 = DG. | last5 = Lin | first5 = YH. | last6 = Van Ziffle | first6 = J. | last7 = Joseph | first7 = NM. | last8 = Onodera | first8 = C. | last9 = Talevich | first9 = E. | title = A recurrent kinase domain mutation in PRKCA defines chordoid glioma of the third ventricle. | journal = Nat Commun | volume = 9 | issue = 1 | pages = 810 | month = 02 | year = 2018 | doi = 10.1038/s41467-018-02826-8 | PMID = 29476136 }}</ref> | |||
<gallery> | |||
File:NP op 20201028 009.jpg | Chordoid Glioma. (WC/jensflorian) | |||
</gallery> | |||
==Ependymal tumours== | ===Ependymal tumours=== | ||
* [[Subependymoma]] | * [[Subependymoma]] | ||
* [[Myxopapillary Ependymoma]] | * [[Myxopapillary Ependymoma]] | ||
| Line 314: | Line 352: | ||
==Other neuroepithelial tumours== | ==Other neuroepithelial tumours== | ||
* [[Neuropathology_tumours#Cribiform_neuroepithelial_tumour|Cribifiorm neuroepithelial tumour]]. | * [[Neuropathology_tumours#Cribiform_neuroepithelial_tumour|Cribifiorm neuroepithelial tumour]]. | ||
===Cribiform neuroepithelial tumour=== | ===Cribiform neuroepithelial tumour=== | ||
| Line 352: | Line 367: | ||
==Neuronal and mixed neuronal/glial tumours== | ==Neuronal and mixed neuronal/glial tumours== | ||
* Desmoplastic infantile astrocytoma / ganglioglioma (DIA/DIG) | * [[Desmoplastic infantile astrocytoma]] / ganglioglioma (DIA/DIG) | ||
* [[Dysembryoplastic neuroepithelial tumour]] | * [[Dysembryoplastic neuroepithelial tumour]] | ||
* [[Central Neurocytoma]] / Extraventricular [[neurocytoma]] | * [[Central Neurocytoma]] / Extraventricular [[neurocytoma]] | ||
* Cerebellar liponeurocytoma | * Cerebellar liponeurocytoma | ||
* [[Papillary glioneuronal tumour]] | * [[Papillary glioneuronal tumour]] (PGNT) | ||
* Rosette-forming glioneuronal tumour of the fourth ventricle | * [[Rosette-forming glioneuronal tumour of the fourth ventricle]] (RGNT) | ||
* Gangliocytoma / Ganglioglioma | * Gangliocytoma / Ganglioglioma | ||
* Dysplastic ganglioglioma of the cerebellum (Lhermitte-Duclos) | * Dysplastic ganglioglioma of the cerebellum ([[Lhermitte-Duclos disease]]) | ||
* [[Paraganglioma]] | * [[Paraganglioma]] | ||
===Desmoplastic infantile astrocytoma / Desmoplastic infantile ganglioglioma=== | |||
* Abbreviated ''DIA'' or ''DIG''. | |||
* ICD-O code: 9412/1 | |||
* Large, superficial, cystic tumor of the infancy. | |||
* Biologic course corresponds to WHO grade I. | |||
* Very rare, included in the WHO since 1993. | |||
* Prominent desmoplastic stroma. | |||
* Astrocytic cells within stroma. | |||
**GFAP+. | |||
**MIB-1 usu. 1%. | |||
* Frequent BRAF V600E or V600D mutations.<ref>{{Cite journal | last1 = Wang | first1 = AC. | last2 = Jones | first2 = DTW. | last3 = Abecassis | first3 = IJ. | last4 = Cole | first4 = BL. | last5 = Leary | first5 = SES. | last6 = Lockwood | first6 = CM. | last7 = Chavez | first7 = L. | last8 = Capper | first8 = D. | last9 = Korshunov | first9 = A. | title = Desmoplastic Infantile Ganglioglioma/Astrocytoma (DIG/DIA) are Distinct Entities with Frequent BRAFV600 Mutations. | journal = Mol Cancer Res | volume = | issue = | pages = | month = Jul | year = 2018 | doi = 10.1158/1541-7786.MCR-17-0507 | PMID = 30006355 }}</ref> | |||
*Single case with BRAF indel or BRAF fusion. | |||
<gallery> | |||
File:DIG-histology.jpg | Histopathology of DIG (HE stain) | |||
File:DIG-histology2.jpg | Prominent ganglioid cells in DIG (HE stain) | |||
</gallery> | |||
===Cerebellar liponeurocytoma=== | ===Cerebellar liponeurocytoma=== | ||
| Line 382: | Line 414: | ||
* NeuN +ve. | * NeuN +ve. | ||
* MIB-1: usu 1-3%. | * MIB-1: usu 1-3%. | ||
====Molecular==== | |||
* Distinct methylation profile. | |||
* Recurent losses on 2p and Chr. 14.<ref>{{Cite journal | last1 = Capper | first1 = D. | last2 = Stichel | first2 = D. | last3 = Sahm | first3 = F. | last4 = Jones | first4 = DTW. | last5 = Schrimpf | first5 = D. | last6 = Sill | first6 = M. | last7 = Schmid | first7 = S. | last8 = Hovestadt | first8 = V. | last9 = Reuss | first9 = DE. | title = Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. | journal = Acta Neuropathol | volume = | issue = | pages = | month = Jul | year = 2018 | doi = 10.1007/s00401-018-1879-y | PMID = 29967940 }}</ref> | |||
<gallery> | <gallery> | ||
| Line 403: | Line 439: | ||
:'''Not''' to be confused with ''[[ganglioneuroma]]''. | :'''Not''' to be confused with ''[[ganglioneuroma]]''. | ||
====General==== | ====General==== | ||
*Grade I WHO mixed neuronal-glial tumour | *Gangliolioma: Grade I WHO mixed neuronal-glial tumour (ICD-O code: 9505/1). | ||
*Anaplastic ganglioglioma: Grade III (ICD-O: 9505/3) | |||
*Rare. | *Rare (approx. 0.5% of all CNS tumors). | ||
*Usu. temporal lobe. | *Usu. temporal lobe. | ||
*Predominantly children (mean age: 9 years). | |||
*Recognized as a cause of [[epilepsy]].<ref name=pmid12125968>{{Cite journal | last1 = Im | first1 = SH. | last2 = Chung | first2 = CK. | last3 = Cho | first3 = BK. | last4 = Lee | first4 = SK. | title = Supratentorial ganglioglioma and epilepsy: postoperative seizure outcome. | journal = J Neurooncol | volume = 57 | issue = 1 | pages = 59-66 | month = Mar | year = 2002 | doi = | PMID = 12125968 }}</ref> | *Recognized as a cause of [[epilepsy]].<ref name=pmid12125968>{{Cite journal | last1 = Im | first1 = SH. | last2 = Chung | first2 = CK. | last3 = Cho | first3 = BK. | last4 = Lee | first4 = SK. | title = Supratentorial ganglioglioma and epilepsy: postoperative seizure outcome. | journal = J Neurooncol | volume = 57 | issue = 1 | pages = 59-66 | month = Mar | year = 2002 | doi = | PMID = 12125968 }}</ref> | ||
*Favourable prognosis (survival rates up to 97%) | |||
**Insufficient data für anaplastic ganglioglioma. | |||
====Macroscopic==== | |||
*Circumscribed lesion. | |||
*Usu. contrast enhancing. | |||
*Solid, but intracortical cysts may be present. | |||
*Little mass effect. | |||
====Microscopic==== | ====Microscopic==== | ||
| Line 417: | Line 463: | ||
**Binucleated (very occassionally). | **Binucleated (very occassionally). | ||
*Atypical glia. | *Atypical glia. | ||
*Eosinophilic granular bodies. | |||
*Calcification. | *Calcification. | ||
*Prominent capillary network. | |||
*Lymphocytic cuffing. | *Lymphocytic cuffing. | ||
*May contain some reticulin. | |||
*Glial component may resemble: | |||
**Fibrillary astrocytoma. | |||
**Oligodendroglioma. | |||
**Pilocytic astrocytoma. | |||
Anaplastic ganglioglioma: | Anaplastic ganglioglioma: | ||
*Brisk mitotic activity | *Brisk mitotic activity | ||
| Line 430: | Line 484: | ||
*Glia: | *Glia: | ||
**CD34+/-ve | **CD34+/-ve | ||
*BRAF V600E +ve (approx. 25%, mainly ganglion cells). | |||
====Molecular==== | |||
*BRAF V600E-mutated(approx. 25%). | |||
*IDH1/2 wt. | |||
*No 1p/19q codeletion. | |||
*Usu. Chr. 7 gain. | |||
*CDKN2A deletions in anaplastic ganglioglioma. | |||
====DDx:==== | ====DDx:==== | ||
| Line 435: | Line 497: | ||
*[[Oligodendroglioma]]. | *[[Oligodendroglioma]]. | ||
*Trapped cortical neurons in diffuse astrocytoma. | *Trapped cortical neurons in diffuse astrocytoma. | ||
*Papillary glioneuronal tumor. | |||
*Dysembryoplastic neuroepithelial tumor. | |||
====Images==== | ====Images==== | ||
| Line 441: | Line 505: | ||
File:Ganglioglioma calcification.jpg | Calcification in ganglioglioma (WC/jensflorian) | File:Ganglioglioma calcification.jpg | Calcification in ganglioglioma (WC/jensflorian) | ||
File:Ganglioglioma Cd34 x200.jpg | CD34 immunostain in ganglioglioma (WC/jensflorian) | File:Ganglioglioma Cd34 x200.jpg | CD34 immunostain in ganglioglioma (WC/jensflorian) | ||
File:Anaplastic ganglioglioma HE.jpg | Pleomorphic ganglion cells in ganglioglioma (WC/jensflorian) | |||
</gallery> | </gallery> | ||
*[http://path.upmc.edu/cases/case142.html Ganglioglioma - case 1 (upmc.edu)]. | *[http://path.upmc.edu/cases/case142.html Ganglioglioma - case 1 (upmc.edu)]. | ||
*[http://path.upmc.edu/cases/case282.html Ganglioglioma - case 2 (upmc.edu)]. | *[http://path.upmc.edu/cases/case282.html Ganglioglioma - case 2 (upmc.edu)]. | ||
===Lhermitte-Duclos disease=== | ===Lhermitte-Duclos disease=== | ||
| Line 451: | Line 515: | ||
*[[AKA]] ''dysplastic gangliocytoma of the cerebellum''. | *[[AKA]] ''dysplastic gangliocytoma of the cerebellum''. | ||
{{Main|Lhermitte-Duclos disease}} | {{Main|Lhermitte-Duclos disease}} | ||
<gallery> | |||
File:Dysplastic_gangliocytoma_lhermitte_duclos.jpg | Dysplastic gangliocytoma (low mag). | |||
</gallery> | |||
===Papillary glioneuronal tumour=== | ===Papillary glioneuronal tumour=== | ||
* Abbreviated ''PGNT''. | * Abbreviated ''PGNT''. | ||
* A benign, supratentorial tumor of childhood. | * A benign, supratentorial tumor of childhood. | ||
** Biologic course corresponds to WHO I | ** Biologic course corresponds to WHO grade I. | ||
** Before WHO 2000, considered a [[Ganglioglioma]] variant. | ** Before WHO 2000, considered a [[Ganglioglioma]] variant. | ||
*Prominent pseudopapillary architecture. | *Prominent pseudopapillary architecture. | ||
| Line 464: | Line 531: | ||
File:PGNT_HE_stain.jpg | PGNT (HE) (WC/jensflorian) | File:PGNT_HE_stain.jpg | PGNT (HE) (WC/jensflorian) | ||
</gallery> | </gallery> | ||
===Rosette-forming glioneuronal tumour of the fourth ventricle=== | |||
* Abbreviated ''RGNT''. | |||
* Provisional ICD-O code: 9509/1 | |||
* A rare benign infratentorial tumour of the midline of children and adults. | |||
* Biologic course corresponds to WHO grade I. | |||
* Glial component corresponds to [[pilocytic astrocytoma]]. | |||
* Neurocytic rosettes. | |||
* Eosinopil fibrillary cores / pseudorosettes. | |||
* GFAP+ in fibrillary areas, Syn+ in rosettes. | |||
* Neurocytic cells: MAP2+ | |||
* MIB-1 usu. below 3%. | |||
<gallery> | |||
File:Histology RGNT HE.jpg | RGNT, HE stain (WC/jensflorian). | |||
File:RGNT HE 2.jpg | RGNT, higher magnification (WC/jensflorian). | |||
</gallery> | |||
===Polymorphous low-grade tumor of the young (PLNTY)=== | |||
* [[Pediatric-type diffuse low-grade glioma#Diffuse low-grade glioma, MAPK pathway-altered|Polymorphous low-grade tumor of the young (PLNTY)]] | |||
==Pineal tumours== | ==Pineal tumours== | ||
| Line 478: | Line 564: | ||
* [[Primitive neuroectodermal tumour]] (PNET) | * [[Primitive neuroectodermal tumour]] (PNET) | ||
* [[Embryonal tumour with abundant neuropil and true rosettes]] (ETANTR) | * [[Embryonal tumour with abundant neuropil and true rosettes]] (ETANTR) | ||
DDx: | |||
* [[Ewing sarcoma]] | |||
* [[Sarcoma with CIC-rearrangement]] | |||
==Peripheral nerve sheath tumours== | ==Peripheral nerve sheath tumours== | ||
| Line 543: | Line 633: | ||
Common pattern: | Common pattern: | ||
*CD20 +ve - key stain. | *[[CD20]] +ve - key stain. | ||
*CD3 -ve. | *CD3 -ve. | ||
*Ki-67 ~40%. | *Ki-67 ~40%. | ||
Latest revision as of 09:04, 14 April 2022
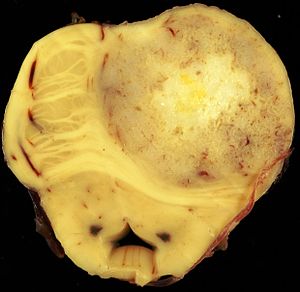
The article covers tumours in neuropathology. Tumours are a large part of neuropathology. Cytopathology of CNS tumours is dealt with in the article CNS cytopathology.
There are separate articles for peripheral nerve sheath tumours and pituitary/peri-pituitary lesions.
Brain tumours - overview
Alphabetical
For overview see here
By age group
Adult
Four most common types of brain tumours:[1]
- Metastatic brain tumours (barely edges out primary tumours)
- Lung (most common).
- Breast.
- Melanoma.
- Renal cell carcinoma (RCC).
- Glioblastoma, IDH-wildtype.
- Astrocytoma, IDH-mutant.
- Meningioma.
Children
- Pilocytic astrocytoma.
- Medulloblastoma.
- Ependymoma.
- Pontine glioma, often Diffuse midline glioma, H3 K27-altered.
By location
Certain tumours like to hang-out at certain places:[2]
Cerebrum
- Cortical based - oligodendroglioma.
- Grey-white junction - metastases.
- White matter - astrocytoma, glioblastoma.
- Periventricular - CNS lymphoma.
- Cystic - ganglioglioma, pilocytic astrocytoma, pleomorphic xanthoastrocytoma.
Cerebellum
- Midline/central - medulloblastoma.
- Cystic lesion - pilocytic astrocytoma (younger individual), hemangioblastoma (older individual).
- Solid lesion (older individual) - metastasis.
Sella turcica
less common:
- Pituicytoma.
- Granular cell tumour.
- Germinoma.
- Chordoma
- Rathke cleft cyst.
- Hypophysitis.
- Xanthogranuloma.
Spinal cord
- Ependymoma
- Glioblastoma
- Meningioma
- Carcinoma metastasis
- Hemangioblastoma
Filum terminale
Meninges
- Meningioma.
- Solitary fibrous tumour / Hemangiopericytoma.
- Hemangioblastoma.
less common:
- Melanoma / Melanocytoma.
- Lymphoproliferative diseases.
- Sarcoidosis
- Arachnoid cyst.
- Disseminated oligodendroglial-like leptomeningeal tumour.
- Desmoplastic infantile astrocytoma / ganglioglioma.
- Meningioangiomatosis.
- Calcifying pseudoneoplasm.
Skull
- Fibrous dysplasia.
- Paget disease.
- Histiocytosis.
- Hemangioma.
- Aneurysmal bone cyst.
- Multiple myeloma.
Skull base / Cerebellopontine angle
- Schwannoma.
- Meningioma.
- Dermoid cyst / epidermoid cyst.
less common:
- Ependymoma.
- Choroid plexus papilloma.
- Glomus tumour.
- Chordoma.
- Chondrosarcoma.
- Olfactory neuroblastoma.
- Endolymphatic sac tumour.
Primary versus secondary
- AKA (primary) brain tumour versus metastatic cancer.
Primary
- Cytoplasmic processes - key feature.
- Best seen at highest magnification - usu. ~1 micrometer.
- Processes may branch.
- Ill-defined border/blend with the surrounding brain.
- Lesion often dura-based.
- Mesenchymal tumor (often contains collagen).
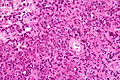
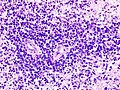
- Primary CNS Lymphoma (PCNSL) is usu. a diffuse large B-cell lymphoma.
- Large (lymphoid) cells, ergo usu. not a difficult diagnosis.
- ~2x size of resting lymphocyte, nucleoli.
- Lesion predominantly perivascular.
Secondary
- Carcinomas:
- Well-demarcated border between brain and lesion - key feature.
- No cytoplasmic processes.
- Usu. have nuclear atypia of malignancy.
- Nuclei often ~3-4x the size of a RBC.
- +/-Glandular arrangement.
- +/-Nucleoli.
- Melanoma.
- Secondary Lymphoma.
- Sarcomas (rare).
By growth pattern
Infiltrative astrocytomas
- Astrocytoma, IDH-mutant.
- Glioblastoma, IDH-wildtype.
Notes:
- Glial: "blends into brain"/gradual transition to non-tumour brain.
Non-infiltrative astrocytomas
Cystic tumours
DDx:[3]
- Pilocytic astrocytoma.
- Pleomorphic xanthoastrocytoma.
- Ganglioglioma.
- Hemangioblastoma.
- Craniopharyngioma.[4]
Notes:
- Non-glial: no radiating glial processes.
- Rosenthal fibres within the tumour... often seen in pilocytic astrocytoma.
- Rosenthal fibres may be seen around a (very) slow growing tumour and represent a reactive process.
- Inflammatory cells and macrophages should prompt consideration of an alternate diagnosis (e.g. cerebral infarct, multiple sclerosis) - esp. if this is a primary lesion.[5]
Grading
Nuclear pleomorphism present:
- At least grade II (diffuse astrocytoma).
Mitotic figures present:
- At least grade III (anaplastic astrocytoma).
Microvascular proliferation or necrosis with pseudopalisading tumour cells:
- Grade IV (glioblastoma AKA glioblastoma multiforme).
Notes:
- Pseudopalisading tumour cells = high tumour cell density adjacent to regions of necrosis; palisade = a fence of poles forming a defensive barrier or fortification.
- WHO Grading is currently based on expected biologiocal behaviour without treatment.
- Grading does not reflect molecular divergent groups within a tumor class or response to therapy (Currently controversies in grading for IDH-mutant astrocytoma vs. IDH-wildtype astrocytoma).[6]
By IHC
- GFAP - should stain cytoplasm of tumour cells and the perikaryon (nuclear membrane) of most Astrocytomas.
- IDH-1(R132H) (isocitrate dehydrogenase 1) in Astrocytoma, IDH-mutant.[7][8]
- H3F3A K27M in Diffuse midline glioma, H3 K27-altered.
- ATRX -ve in Astrocytoma, IDH-mutant or Diffuse hemispheric glioma, H3 G34-mutant.
- CD20 in PCNSL.
- Cytokeratins in Carcinoma brain metastases, Plexus choroid tumours, AT/RT, Papillary tumour of the pineal region, Craniopharyngioma.
- EMA in Meningioma and carcinoma brain metastases.
- PrgR in Meningioma and carcinoma metastases.
- Synaptophysin in glioneuronal tumours and Pituitary adenoma and embryonal tumours.
Common neuropathology tumours in a table
| Type | Key feature(s) | Imaging | History | Notes | IHC | Images |
| Normal tissue | cells regularly spaced, no nuc. atypia | small lesion? / deep lesion? | variable | missed lesion? | nil | |
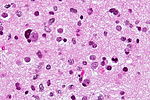
| Reactive astrocytes | astrocytes with well-demarcated eosinophilic cytoplasm, regular spacing, no nuc. atypia | small lesion? / deep lesion? | variable | missed lesion / close to a lesion; non-specific pathologic process - need more tissue | GFAP | |
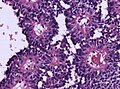
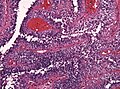
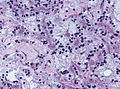
| Schwannoma | cellular areas (Antoni A), paucicelluar areas (Antoni B), palisading of nuclei (Verocay bodies) | extra-axial + intradural | old or young | need frozen section to Dx, DDx: meningioma | S100, SOX10 | |
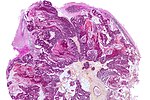
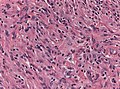
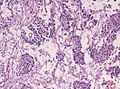
| Meningioma | whorls, psammomatous calcs, nuclear inclusions | extra-axial + intradural | old or young | may be diagnosed on smear, DDx: schwannoma, choroid plexus | EMA, PR, Ki-67 | |
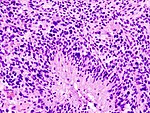
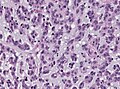
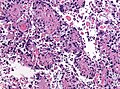
| Astrocytoma, IDH-mutant (CNS WHO grade 2 or grade 3) | glial processes (esp. on smear), nuclear atypia (typical size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion †, no microvascular proliferation, no necrosis | often enhancing (suggests high grade), usu. supratentorial, usu. white matter | usu. old, occ. young | common | IDH-1(R132H)+/-, GFAP+ | |
| Glioblastoma, IDH-wildtype (CNS WHO grade 4) | glial processes (esp. on smear), nuclear atypia (typical size var. ~3x, irreg. nuc. membrane, hyperchromasia), no Rosenthal fibres in the core of the lesion †, microvascular proliferation or necrosis | often enhancing (suggests high grade), usu. supratentorial, usu. white matter | usu. old, occ. young | very common, esp. glioblastoma | IDH-1+/-, GFAP+ | |
| Metastasis | sharp interface with brain, often glandular, +/-nucleoli, no glial processes | often cerebellular, well-circumscribed | usu. old | often suspected to have metastatic disease | TTF-1, CK7, CK20, BRST-2 |
† Rosenthal fibres at the periphery of a lesion are a non-specific finding seen in chronic processes.
Brain metastasis
Molecular
See also: Molecular Neuropathology
Gliomas
Gliomas, glioneuronal tumours and neuronal tumours are often categorized together.
Astrocytic tumours
- Astrocytoma, IDH-mutant.
- Glioblastoma, IDH-wildtype.
- Gliosarcoma (a glioblastoma subtype)
- Glial processes - key feature.
- Thin stringy cytoplasmic processes - best seen at high power in less cellular areas.
- No Rosenthal fibres within the tumour itself.
Images:
- Endothelial proliferation in a GBM (ouhsc.edu).
- Endothelial proliferation (ouhse.edu).
- Gemistocytic astrocytoma - several images (upmc.edu).
Depreceated:
- Diffuse Astrocytoma
- Anaplastic astrocytoma
- Gliomatosis cerebri
- Spongioblastoma
Oligodendroglial tumours
- Oligodendroglioma, IDH-mutant and 1p/19q codeleted.
Depreceated:
- Anaplastic oligodendroglioma
- Oligoastrocytoma
- Anaplastic oligoastrocytoma
Pediatric-type diffuse high-grade glioma
Pediatric-type diffuse low-grade glioma
Circumscribed astrocytic gliomas
- Pilocytic astrocytoma (PA)
- Pilomyxoid astrocytoma (PMA)
- Pleomorphic xanthoastrocytoma (PXA)
- Subependymal giant cell astrocytoma (SEGA)
- Astroblastoma MN1-altered.
- Chordoid glioma.
Astroblastoma
- No WHO grade yet.[11]
- Very rare superficial tumor of young age.[12]
- Large, cystic. Pushing margin towards CNS.
- Vasocentric growth, plump cells with absence of fibrillary pattern.
- GFAP+ve, Synaptohysin-ve, Olig-2-ve, focally EMA/panCK+ve. MIB-1: 1-18 %.
- Molecular profile overlaps with classical CNS-PNET.
- Gene fusions invoving meningioma gene (MN1)[13]
Chordoid glioma of the third ventricle
- WHO grade II.
- Slowly growing, non-invasive, in adults.
- Clusters of epithelioid cells in mucinous stroma.
- Lymphocytic infiltrates, adjacent Rosenthal fibers.
- Fibrosis may be present.
- Few mitoses.
- GFAP+ve, MIB-1 1-3%.
- TTF-1+ve.
- CD34+ve.
- IDH-1-ve, p53-ve.
- PRKCA D463H mutations.[14]
Ependymal tumours
- Subependymoma
- Myxopapillary Ependymoma
- Ependymoma
- Anaplastic ependymoma
Choroid plexus tumours
- Choroid plexus papilloma
- Atypical choroid plexus papilloma
- Choroid plexus carcinoma
Other neuroepithelial tumours
Cribiform neuroepithelial tumour
AKA: CRINET.
- Not listed in the current WHO classification.
- First description in 2009.[15]
- Around ventricles.[16]
- Young children.[17]
- Small undifferentiated cells arranged in cribriform strands and trabeculae of varying thickness.
- MAP2+ve, Synaptophysin+ve, CK+/-ve. MIB-1: 30%.
- INI-1 loss, but no rhabdoid features and good prognosis.
- Stable genomic profile.[18]
Neuronal and mixed neuronal/glial tumours
- Desmoplastic infantile astrocytoma / ganglioglioma (DIA/DIG)
- Dysembryoplastic neuroepithelial tumour
- Central Neurocytoma / Extraventricular neurocytoma
- Cerebellar liponeurocytoma
- Papillary glioneuronal tumour (PGNT)
- Rosette-forming glioneuronal tumour of the fourth ventricle (RGNT)
- Gangliocytoma / Ganglioglioma
- Dysplastic ganglioglioma of the cerebellum (Lhermitte-Duclos disease)
- Paraganglioma
Desmoplastic infantile astrocytoma / Desmoplastic infantile ganglioglioma
- Abbreviated DIA or DIG.
- ICD-O code: 9412/1
- Large, superficial, cystic tumor of the infancy.
- Biologic course corresponds to WHO grade I.
- Very rare, included in the WHO since 1993.
- Prominent desmoplastic stroma.
- Astrocytic cells within stroma.
- GFAP+.
- MIB-1 usu. 1%.
- Frequent BRAF V600E or V600D mutations.[19]
- Single case with BRAF indel or BRAF fusion.
Cerebellar liponeurocytoma
- Previously called lipomatous medulloblastoma (name changed in WHO 2000).
- Mean age: 50 years.
- As the name states: A tumour of the cerebellum.
- But cases outside cerebellum reported that would qualify.[20]
- WHO grade II [21] (upgraded from WHO grade I in 2007)[22]
- ICD-O code: 9506/1
Histo
- Advanced neuronal and lipomatous differentiation.
- Neurocytes: round to oval nuclei with clear cytoplasm.
- Quite cellular.
- Mitoses almost absent.
IHC
Molecular
- Distinct methylation profile.
- Recurent losses on 2p and Chr. 14.[23]
DDx
Gangliocytoma
- Grade I WHO neuronal tumour.
- ICD-O code: 9492/0
- Groups of irregular large neurons.
- Non-neoplastic, reticulin-rich glial stroma.
Ganglioglioma
- Not to be confused with ganglioneuroma.
General
- Gangliolioma: Grade I WHO mixed neuronal-glial tumour (ICD-O code: 9505/1).
- Anaplastic ganglioglioma: Grade III (ICD-O: 9505/3)
- Rare (approx. 0.5% of all CNS tumors).
- Usu. temporal lobe.
- Predominantly children (mean age: 9 years).
- Recognized as a cause of epilepsy.[24]
- Favourable prognosis (survival rates up to 97%)
- Insufficient data für anaplastic ganglioglioma.
Macroscopic
- Circumscribed lesion.
- Usu. contrast enhancing.
- Solid, but intracortical cysts may be present.
- Little mass effect.
Microscopic
Features:
- Dysplastic neurons.
- Out of regular architecture / abnormal location.
- Cytomegaly
- Clustering
- Binucleated (very occassionally).
- Atypical glia.
- Eosinophilic granular bodies.
- Calcification.
- Prominent capillary network.
- Lymphocytic cuffing.
- May contain some reticulin.
- Glial component may resemble:
- Fibrillary astrocytoma.
- Oligodendroglioma.
- Pilocytic astrocytoma.
Anaplastic ganglioglioma:
- Brisk mitotic activity
- Necrosis
IHC
- Neurons:
- MAP2 +ve
- Synaptophysin +ve
- Neurofilament +ve
- Glia:
- CD34+/-ve
- BRAF V600E +ve (approx. 25%, mainly ganglion cells).
Molecular
- BRAF V600E-mutated(approx. 25%).
- IDH1/2 wt.
- No 1p/19q codeletion.
- Usu. Chr. 7 gain.
- CDKN2A deletions in anaplastic ganglioglioma.
DDx:
- DNT.
- Oligodendroglioma.
- Trapped cortical neurons in diffuse astrocytoma.
- Papillary glioneuronal tumor.
- Dysembryoplastic neuroepithelial tumor.
Images
Lhermitte-Duclos disease
- Abbreviated LDD.
- AKA dysplastic cerebellar gangliocytoma.[25]
- AKA dysplastic gangliocytoma of the cerebellum.
Papillary glioneuronal tumour
- Abbreviated PGNT.
- A benign, supratentorial tumor of childhood.
- Biologic course corresponds to WHO grade I.
- Before WHO 2000, considered a Ganglioglioma variant.
- Prominent pseudopapillary architecture.
- Neurocytes to medium-sized ganglion cells.
- GFAP+ core, GFAP- layer
- Rosenthal fibers, Eosinophilic Granular bodies and lymphocytic cuffing may be present.
Rosette-forming glioneuronal tumour of the fourth ventricle
- Abbreviated RGNT.
- Provisional ICD-O code: 9509/1
- A rare benign infratentorial tumour of the midline of children and adults.
- Biologic course corresponds to WHO grade I.
- Glial component corresponds to pilocytic astrocytoma.
- Neurocytic rosettes.
- Eosinopil fibrillary cores / pseudorosettes.
- GFAP+ in fibrillary areas, Syn+ in rosettes.
- Neurocytic cells: MAP2+
- MIB-1 usu. below 3%.
Polymorphous low-grade tumor of the young (PLNTY)
Pineal tumours
- Pineocytoma
- Pineal parenchymal tumour of intermediate differentiation
- Pineoblastoma
- Papillary tumour of the pineal region
Embryonal tumours
- Atypical teratoid/rhabdoid tumour (AT/RT) or (AT-RT)
- Medulloblastoma
- Primitive neuroectodermal tumour (PNET)
- Embryonal tumour with abundant neuropil and true rosettes (ETANTR)
DDx:
Peripheral nerve sheath tumours
A classification:[26] Benign:
- Schwannoma.
- Neurofibroma.
- Perineurioma.
- Ganglioneuroma.
Malignant:
Ganglioneuroma
- Not to be confused with ganglioglioma.
Meningioma
Chordoma
Hemangioblastoma
CNS lymphoma
Classification:
- Primary CNS lymphoma.
- Non-primary CNS lymphoma - see lymphoma article.
General - primary CNS
- Classically periventicular distribution.
- Usually large B cell; can be considered a type of diffuse large B cell lymphoma (DLBCL).
- Prognosis of CNS (DLBCL) lymphomas worse than nodal (non-CNS) DLBCL.[28]
Microscopic
Features:
- Large cell lymphoma.
- Size = 2x diameter normal lymphocyte.
- Nucleolus - common.
- Perivascular clustering.
Images
www:
IHC
Can be subclassified in GCB (germinal centre B-cell-like) and non-GCB by CD10, Bcl-6, MUM1/IRF-4, and Bcl-2.[28]
Common pattern:
- CD20 +ve - key stain.
- CD3 -ve.
- Ki-67 ~40%.
- Bcl-6 +ve.
- Bcl-1 -ve.
Ganglioneuroblastoma
General
- Uncommon.
- Part of the neuroblastic tumours group which includes:[29]
- Ganglioneuroma (benign).
- Ganglioneuroblastoma (intermediate).
- Neuroblastoma (aggressive).
Microscopic
Features:
- Ganglion-like cells with a prominent nucleolus.
- Small undifferentiated cells with scant cytoplasm.
Images:
IHC
- NSE +ve -- small cells.
Lesions of the sella turcica
Lesions of the sella turcica, the pituitary gland environs, is a topic for it self. The differential diagnosis for lesions in this area includes:
- Pituitary adenoma.
- Craniopharyngioma.
- Rathke cleft cyst.
- Germ cell tumour.
- Meningioma.
- Pilomyxoid astrocytoma - in children.
See also
References
- ↑ http://neurosurgery.mgh.harvard.edu/abta/primer.htm
- ↑ URL: http://www.msdlatinamerica.com/ebooks/DiagnosticNeuropathologySmears/files/4ce563fb7e8e48fc9ed8b42e296a7747.gif and http://www.msdlatinamerica.com/ebooks/DiagnosticNeuropathologySmears/sid117213.html. Accessed on: 2 November 2010.
- ↑ URL: http://path.upmc.edu/cases/case320/dx.html. Accessed on: 14 January 2012.
- ↑ URL: http://www.pathologyoutlines.com/Cnstumor.html#cystsgeneral. Accessed on: 14 January 2012.
- ↑ URL: http://path.upmc.edu/cases/case79/dx.html. Accessed on: 2 January 2012.
- ↑ Louis, DN.; von Deimling, A. (Aug 2017). "Grading of diffuse astrocytic gliomas: Broders, Kernohan, Zülch, the WHO… and Shakespeare.". Acta Neuropathol. doi:10.1007/s00401-017-1765-z. PMID 28801693.
- ↑ Yan H, Parsons DW, Jin G, et al. (February 2009). "IDH1 and IDH2 mutations in gliomas". N. Engl. J. Med. 360 (8): 765–73. doi:10.1056/NEJMoa0808710. PMC 2820383. PMID 19228619. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2820383/.
- ↑ Houillier C, Wang X, Kaloshi G, et al. (October 2010). "IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas". Neurology 75 (17): 1560–6. doi:10.1212/WNL.0b013e3181f96282. PMID 20975057.
- ↑ Rong Y, Durden DL, Van Meir EG, Brat DJ (June 2006). "'Pseudopalisading' necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis". J. Neuropathol. Exp. Neurol. 65 (6): 529–39. PMID 16783163.
- ↑ http://dictionary.reference.com/browse/palisading
- ↑ The International Agency for Research on Cancer (Editors: Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.) (2007). Pathology and Genetics of Tumours of Tumors of the Central Nervous System (IARC WHO Classification of Tumours) (4th ed.). Lyon: World Health Organization. pp. 88. doi:10.1007/s00401-007-0243-4. ISBN 978-9283224303.
- ↑ Narayan, S.; Kapoor, A.; Singhal, MK.; Jakhar, SL.; Bagri, PK.; Rajput, PS.; Kumar, HS.. "Astroblastoma of cerebrum: A rare case report and review of literature.". J Cancer Res Ther 11 (3): 667. doi:10.4103/0973-1482.140800. PMID 26458709.
- ↑ Sturm, D.; Orr, BA.; Toprak, UH.; Hovestadt, V.; Jones, DT.; Capper, D.; Sill, M.; Buchhalter, I. et al. (Feb 2016). "New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs.". Cell 164 (5): 1060-72. doi:10.1016/j.cell.2016.01.015. PMID 26919435.
- ↑ Goode, B.; Mondal, G.; Hyun, M.; Ruiz, DG.; Lin, YH.; Van Ziffle, J.; Joseph, NM.; Onodera, C. et al. (02 2018). "A recurrent kinase domain mutation in PRKCA defines chordoid glioma of the third ventricle.". Nat Commun 9 (1): 810. doi:10.1038/s41467-018-02826-8. PMID 29476136.
- ↑ Hasselblatt, M.; Oyen, F.; Gesk, S.; Kordes, U.; Wrede, B.; Bergmann, M.; Schmid, H.; Frühwald, MC. et al. (Dec 2009). "Cribriform neuroepithelial tumor (CRINET): a nonrhabdoid ventricular tumor with INI1 loss and relatively favorable prognosis.". J Neuropathol Exp Neurol 68 (12): 1249-55. doi:10.1097/NEN.0b013e3181c06a51. PMID 19915490.
- ↑ Arnold, MA.; Stallings-Archer, K.; Marlin, E.; Grondin, R.; Olshefski, R.; Biegel, JA.; Pierson, CR.. "Cribriform neuroepithelial tumor arising in the lateral ventricle.". Pediatr Dev Pathol 16 (4): 301-7. doi:10.2350/12-12-1287-CR.1. PMID 23495723.
- ↑ Park, JY.; Kim, E.; Kim, DW.; Chang, HW.; Kim, SP. (Oct 2012). "Cribriform neuroepithelial tumor in the third ventricle: a case report and literature review.". Neuropathology 32 (5): 570-6. doi:10.1111/j.1440-1789.2011.01293.x. PMID 22239490.
- ↑ Gessi, M.; Japp, AS.; Dreschmann, V.; Zur Mühlen, A.; Goschzik, T.; Dörner, E.; Pietsch, T. (Oct 2015). "High-Resolution Genomic Analysis of Cribriform Neuroepithelial Tumors of the Central Nervous System.". J Neuropathol Exp Neurol 74 (10): 970-4. doi:10.1097/NEN.0000000000000239. PMID 26352987.
- ↑ Wang, AC.; Jones, DTW.; Abecassis, IJ.; Cole, BL.; Leary, SES.; Lockwood, CM.; Chavez, L.; Capper, D. et al. (Jul 2018). "Desmoplastic Infantile Ganglioglioma/Astrocytoma (DIG/DIA) are Distinct Entities with Frequent BRAFV600 Mutations.". Mol Cancer Res. doi:10.1158/1541-7786.MCR-17-0507. PMID 30006355.
- ↑ Gupta, K.; Salunke, P.; Kalra, I.; Vasishta, RK.. "Central liponeurocytoma: case report and review of literature.". Clin Neuropathol 30 (2): 80-5. PMID 21329617.
- ↑ Nishimoto, T.; Kaya, B. (Aug 2012). "Cerebellar liponeurocytoma.". Arch Pathol Lab Med 136 (8): 965-9. doi:10.5858/arpa.2011-0337-RS. PMID 22849747.
- ↑ Brat, DJ.; Parisi, JE.; Kleinschmidt-DeMasters, BK.; Yachnis, AT.; Montine, TJ.; Boyer, PJ.; Powell, SZ.; Prayson, RA. et al. (Jun 2008). "Surgical neuropathology update: a review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition.". Arch Pathol Lab Med 132 (6): 993-1007. doi:10.1043/1543-2165(2008)132[993:SNUARO]2.0.CO;2. PMID 18517285.
- ↑ Capper, D.; Stichel, D.; Sahm, F.; Jones, DTW.; Schrimpf, D.; Sill, M.; Schmid, S.; Hovestadt, V. et al. (Jul 2018). "Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience.". Acta Neuropathol. doi:10.1007/s00401-018-1879-y. PMID 29967940.
- ↑ Im, SH.; Chung, CK.; Cho, BK.; Lee, SK. (Mar 2002). "Supratentorial ganglioglioma and epilepsy: postoperative seizure outcome.". J Neurooncol 57 (1): 59-66. PMID 12125968.
- ↑ Yağci-Küpeli, B.; Oguz, KK.; Bilen, MA.; Yalçin, B.; Akalan, N.; Büyükpamukçu, M. (Mar 2010). "An unusual cause of posterior fossa mass: Lhermitte-Duclos disease.". J Neurol Sci 290 (1-2): 138-41. doi:10.1016/j.jns.2009.12.010. PMID 20060133.
- ↑ Wippold FJ, Lubner M, Perrin RJ, Lämmle M, Perry A (October 2007). "Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns". AJNR Am J Neuroradiol 28 (9): 1633–8. doi:10.3174/ajnr.A0682. PMID 17893219. http://www.ajnr.org/cgi/reprint/28/9/1633.
- ↑ URL: http://medical-dictionary.thefreedictionary.com/ganglioma. Accessed on: 8 November 2010.
- ↑ 28.0 28.1 Raoux D, Duband S, Forest F, et al. (June 2010). "Primary central nervous system lymphoma: Immunohistochemical profile and prognostic significance". Neuropathology 30 (3): 232–40. doi:10.1111/j.1440-1789.2009.01074.x. PMID 19925562.
- ↑ Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B (July 1999). "Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee". Cancer 86 (2): 349–63. PMID 10421272.