Difference between revisions of "Prostate cancer"
m (vauthors) |
|||
| (218 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{ Infobox diagnosis | |||
| Name = Prostate carcinoma | |||
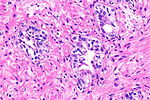
| Image = Prostate cancer with Gleason pattern 4 low mag.jpg | |||
| Width = | |||
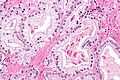
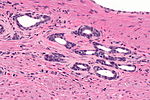
| Caption = Prostate carcinoma. [[H&E stain]]. | |||
| Micro = major criteria: abnormal architecture (increased gland density, usu. small circular glands, "infiltrative growth" pattern), basal cells lost, cytological abnormalities (nuclear enlargement, nucleoli); minor criteria: nuclear hyperchromasia, wispy blue mucin, pink amorphous secretions, intraluminal crystalloid, amphophilic cytoplasm, adjacent [[HGPIN]], mitoses | |||
| Subtypes = | |||
| LMDDx = [[high-grade prostatic intraepithelial neoplasia]], [[atypical small acinar proliferation]] (biopsy only), [[prostatic atrophy]], [[seminal vesicle]], [[basal cell hyperplasia of the prostate|basal cell hyperplasia]], others | |||
| Stains = | |||
| IHC = PSA +ve, PSAP +ve, AMACR +ve, p63 -ve, CK34betaE12 -ve | |||
| EM = | |||
| Molecular = +/-[[BRCA1]] mutation (genetic predisposition), +/-[[BRCA2]] mutation (genetic predisposition) | |||
| IF = | |||
| Gross = usu. posterior aspect of the prostate - often not apparent at gross | |||
| Grossing = [[prostate biopsy]], [[prostate chips]], [[radical prostatectomy]] | |||
| Staging = [[prostate cancer staging]] | |||
| Site = [[prostate gland]] | |||
| Assdx = | |||
| Syndromes = | |||
| Clinicalhx = | |||
| Signs = firm, nodular prostate on digital rectal exam | |||
| Symptoms = often asymptomatic | |||
| Prevalence = very common | |||
| Bloodwork = PSA elevated (common) | |||
| Rads = hypoechoic areas, no apparent abnormality | |||
| Endoscopy = | |||
| Prognosis = good-to-poor (depends on [[prostate cancer grading|grade (Gleason score)]] and [[stage]]) | |||
| Other = | |||
| ClinDDx = [[prostatitis]], [[nodular hyperplasia of the prostate]] | |||
| Tx = observation (common for low-grade, low tumour burden), radiation or radical prostatectomy | |||
}} | |||
This article deals with '''prostate [[cancer]]'''. | This article deals with '''prostate [[cancer]]'''. | ||
| Line 8: | Line 39: | ||
==General== | ==General== | ||
*Very common. | *Very common. | ||
*Increasing incidence with age - the age in years is an approximation of the percentage of men with prostate cancer. | *Increasing incidence with age - the age in years is an approximation of the percentage of men with prostate cancer.<ref>{{cite journal |author=Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD |title=The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients |journal=J. Urol. |volume=150 |issue=2 Pt 1 |pages=379–85 |year=1993 |month=August |pmid=8326560 |doi= |url=}}</ref>{{fact}} | ||
*Usually an indolent course - most old men die with prostate cancer ''not'' from prostate cancer. | *Usually an indolent course - most old men die with prostate cancer ''not'' from prostate cancer. | ||
*Risk increased with a [[BRCA1]] or [[BRCA2]] mutation<ref name=pmid23747895>{{Cite journal | last1 = Li | first1 = D. | last2 = Kumaraswamy | first2 = E. | last3 = Harlan-Williams | first3 = LM. | last4 = Jensen | first4 = RA. | title = The role of BRCA1 and BRCA2 in prostate cancer. | journal = Front Biosci (Landmark Ed) | volume = 18 | issue = | pages = 1445-59 | month = | year = 2013 | doi = | PMID = 23747895 }}</ref> - families have a mix of [[breast cancer]] and prostate cancer. | |||
**BRCA2 mutation risk >8x for men over 65 years old.<ref name=pmid22522501>{{Cite journal | last1 = Castro | first1 = E. | last2 = Eeles | first2 = R. | title = The role of BRCA1 and BRCA2 in prostate cancer. | journal = Asian J Androl | volume = 14 | issue = 3 | pages = 409-14 | month = May | year = 2012 | doi = 10.1038/aja.2011.150 | PMID = 22522501 }}</ref> | |||
**A BRCA2 founder mutation is described in French Canadians.<Ref name=pmid23318356>{{Cite journal | last1 = Taherian | first1 = N. | last2 = Hamel | first2 = N. | last3 = Bégin | first3 = LR. | last4 = Bismar | first4 = TA. | last5 = Goldgar | first5 = DE. | last6 = Feng | first6 = BJ. | last7 = Foulkes | first7 = WD. | title = Familial prostate cancer: the damage done and lessons learnt. | journal = Nat Rev Urol | volume = 10 | issue = 2 | pages = 116-22 | month = Feb | year = 2013 | doi = 10.1038/nrurol.2012.257 | PMID = 23318356 }}</ref> | |||
===Management=== | ===Management=== | ||
====Dirty first approximation==== | ====Dirty first approximation==== | ||
*The management changes between [[Gleason score]] 6, 7 and 8. | *The management changes between [[Gleason score]] 6, 7 (3+4), 7 (4+3) and 8. | ||
Typically, the implications are: | Typically, the implications are: | ||
* Gleason 6: observation ''or'' radioactive seeds; surgery if patient wants. | * Gleason 6: observation ''or'' radioactive seeds; surgery if patient wants. | ||
* Gleason 7: do something -- surgery ''or'' radiation therapy. | * Gleason 7 with a bit of Gleason pattern 4 and a low tumour volume: it is reasonable to watch ''or'' do something. ‡ | ||
* Gleason 7 with a lot of Gleason pattern 4 ''or'' a high tumour volume: do something -- surgery ''or'' radiation therapy. | |||
* Gleason 8+: bad cancer -- do something quickly! | * Gleason 8+: bad cancer -- do something quickly! | ||
Note: | |||
* ‡ It has been said that ''Gleason score 7 with a bit of Gleason pattern 4 is the new Gleason score 6''. | |||
Bottom line: | Bottom line: | ||
| Line 25: | Line 64: | ||
====Observational strategies==== | ====Observational strategies==== | ||
*Delay of definitive treatment (surgery ''or'' radiation). | *Delay of definitive treatment (surgery ''or'' radiation). | ||
*Common in management of prostate cancer. | *Common in the management of prostate cancer. | ||
Classification:<ref name=pmid23126653>{{Cite journal | last1 = Ip | first1 = S. | last2 = Dahabreh | first2 = IJ. | last3 = Chung | first3 = M. | last4 = Yu | first4 = WW. | last5 = Balk | first5 = EM. | last6 = Iovin | first6 = RC. | last7 = Mathew | first7 = P. | last8 = Luongo | first8 = T. | last9 = Dvorak | first9 = T. | title = An evidence review of active surveillance in men with localized prostate cancer. | journal = Evid Rep Technol Assess (Full Rep) | volume = | issue = 204 | pages = 1-341 | month = Dec | year = 2011 | doi = | PMID = 23126653 | url = http://www.ncbi.nlm.nih.gov/books/NBK83054/ }}</ref> | Classification:<ref name=pmid23126653>{{Cite journal | last1 = Ip | first1 = S. | last2 = Dahabreh | first2 = IJ. | last3 = Chung | first3 = M. | last4 = Yu | first4 = WW. | last5 = Balk | first5 = EM. | last6 = Iovin | first6 = RC. | last7 = Mathew | first7 = P. | last8 = Luongo | first8 = T. | last9 = Dvorak | first9 = T. | title = An evidence review of active surveillance in men with localized prostate cancer. | journal = Evid Rep Technol Assess (Full Rep) | volume = | issue = 204 | pages = 1-341 | month = Dec | year = 2011 | doi = | PMID = 23126653 | url = http://www.ncbi.nlm.nih.gov/books/NBK83054/ }}</ref> | ||
| Line 41: | Line 80: | ||
*Gleason score 6 or less. | *Gleason score 6 or less. | ||
*All biopsies cores < 50% involvement. | *All biopsies cores < 50% involvement. | ||
* | *One or two cores involved.<ref>URL: [http://www.active-surveillance.com/laurence-klotz-md/ http://www.active-surveillance.com/laurence-klotz-md/]. Accessed on: 12 July 2013.</ref><ref name=pmid23548978>{{Cite journal | last1 = Klotz | first1 = L. | title = Active surveillance: patient selection. | journal = Curr Opin Urol | volume = 23 | issue = 3 | pages = 239-44 | month = May | year = 2013 | doi = 10.1097/MOU.0b013e32835f8f6b | PMID = 23548978 }}</ref><ref name=pmid22891078>{{Cite journal | last1 = Klotz | first1 = L. | title = Active surveillance for low-risk prostate cancer. | journal = F1000 Med Rep | volume = 4 | issue = | pages = 16 | month = | year = 2012 | doi = 10.3410/M4-16 | PMID = 22891078 | PMC = 3412317 }}</ref> | ||
Clinical criteria: | |||
*PSA <= 10 ng/mL.<ref name=pmid22314081/> | |||
*Negative DRE. | |||
==Gross== | ==Gross== | ||
*Prostate cancer is uncommonly apparent | *Prostate cancer is uncommonly apparent on gross. | ||
*Classic location: posterior aspect of the prostate. | |||
===Radiology=== | |||
*Hypoechoic areas = suspicious for cancer. | |||
**It seems that size of the area matters. | |||
***Small hypoechoic areas (<0.2 cm<sup>3</sup>) have cancer less than 4% of the time.<ref name=pmid9933054>{{Cite journal | last1 = Fleshner | first1 = NE. | last2 = O'Sullivan | first2 = M. | last3 = Premdass | first3 = C. | last4 = Fair | first4 = WR. | title = Clinical significance of small (less than 0.2 cm3) hypoechoic lesions in men with normal digital rectal examinations and prostate-specific antigen levels less than 10 ng/mL. | journal = Urology | volume = 53 | issue = 2 | pages = 356-8 | month = Feb | year = 1999 | doi = | PMID = 9933054 }}</ref> | |||
***One study suggests hypoechoic lesions tend to have a worse outcome;<ref name=pmid22920545>{{Cite journal | last1 = Nakano Junqueira | first1 = VC. | last2 = Zogbi | first2 = O. | last3 = Cologna | first3 = A. | last4 = Dos Reis | first4 = RB. | last5 = Tucci | first5 = S. | last6 = Reis | first6 = LO. | last7 = Westphalen | first7 = AC. | last8 = Muglia | first8 = VF. | title = Is a visible (hypoechoic) lesion at biopsy an independent predictor of prostate cancer outcome? | journal = Ultrasound Med Biol | volume = 38 | issue = 10 | pages = 1689-94 | month = Oct | year = 2012 | doi = 10.1016/j.ultrasmedbio.2012.06.006 | PMID = 22920545 }}</ref> however, this is not supported by an older study.<ref name=pmid1688955>{{Cite journal | last1 = Devonec | first1 = M. | last2 = Fendler | first2 = JP. | last3 = Monsallier | first3 = M. | last4 = Mouriquand | first4 = P. | last5 = Maquet | first5 = JH. | last6 = Mestas | first6 = JL. | last7 = Dutrieux-Berger | first7 = N. | last8 = Perrin | first8 = P. | title = The significance of the prostatic hypoechoic area: results in 226 ultrasonically guided prostatic biopsies. | journal = J Urol | volume = 143 | issue = 2 | pages = 316-9 | month = Feb | year = 1990 | doi = | PMID = 1688955 }}</ref> | |||
===Prostatectomy grossing=== | ===Prostatectomy grossing=== | ||
{{Main|Radical prostatectomy}} | |||
* | |||
===Cytoprostatectomy grossing=== | |||
{{Main|Cystoprostatectomy grossing}} | |||
*Limited sampling of the prostate may lead to undersampling error.<ref name=pmid11182038>{{Cite journal | last1 = Cindolo | first1 = L. | last2 = Benincasa | first2 = G. | last3 = Autorino | first3 = R. | last4 = Domizio | first4 = S. | last5 = De Rosa | first5 = G. | last6 = Testa | first6 = G. | last7 = D'Armiento | first7 = M. | last8 = Altieri | first8 = V. | title = Prevalence of silent prostatic adenocarcinoma in 165 patients undergone cystoprostatectomy: a retrospective study. | journal = Oncol Rep | volume = 8 | issue = 2 | pages = 269-71 | month = | year = | doi = | PMID = 11182038 }}</ref> | |||
==Microscopic== | ==Microscopic== | ||
| Line 61: | Line 113: | ||
#Basal cells lacking. | #Basal cells lacking. | ||
#Cytological abnormalities: | #Cytological abnormalities: | ||
#*Nuclear enlargement. | #*Nuclear enlargement (subtle). | ||
#*Nucleoli. | #*[[Nucleoli]] (prominent). | ||
Minor criteria:<ref name=pmid17213347/> | Minor criteria:<ref name=pmid17213347/> | ||
| Line 82: | Line 134: | ||
**Thus, it has been suggested that six or more glands should be present to diagnose cancer.<ref name=pmid20061936/> | **Thus, it has been suggested that six or more glands should be present to diagnose cancer.<ref name=pmid20061936/> | ||
===Low power features=== | Features considered pathognomonic for prostate carcinoma by some authorities:<ref name=pmid16613324>{{Cite journal | last1 = Egevad | first1 = L. | last2 = Allsbrook | first2 = WC. | last3 = Epstein | first3 = JI. | title = Current practice of diagnosis and reporting of prostate cancer on needle biopsy among genitourinary pathologists. | journal = Hum Pathol | volume = 37 | issue = 3 | pages = 292-7 | month = Mar | year = 2006 | doi = | PMID = 16613324 }}</ref><ref name=pmid10435561>{{Cite journal | last1 = Baisden | first1 = BL. | last2 = Kahane | first2 = H. | last3 = Epstein | first3 = JI. | title = Perineural invasion, mucinous fibroplasia, and glomerulations: diagnostic features of limited cancer on prostate needle biopsy. | journal = Am J Surg Pathol | volume = 23 | issue = 8 | pages = 918-24 | month = Aug | year = 1999 | doi = | PMID = 10435561 }}</ref> | ||
#Perineural invasion. | |||
#*Must be circumferential (>95% of circumference{{fact}}). | |||
#Glomeruloid bodies. | |||
#[[Collagenous micronodules]] also known as ''mucinous fibroplasia''. | |||
<gallery> | |||
Image: Intraluminal eosinophilic crystalloid of prostate gland - high mag.jpg| Intraluminal eosinophilic crystalloid - high mag. (WC) | |||
Image: Prostate carcinoma with blue mucin -- very high mag.jpg | Whispy blue mucin - very high mag. (WC) | |||
Image: Prostate carcinoma with blue mucin - a1 -- intermed mag.jpg | Whispy blue mucin - intermed. mag. (WC) | |||
</gallery> | |||
===Divided into high and low power=== | |||
====Low power features==== | |||
*Architecture is the '''key''' to diagnosing low grade cancer. | *Architecture is the '''key''' to diagnosing low grade cancer. | ||
**Back-to-back glands or crowding of glands -- think low grade cancer (Gleason pattern 3). | **Back-to-back glands or crowding of glands -- think low grade cancer (Gleason pattern 3). | ||
| Line 91: | Line 156: | ||
**"Infiltrative": small round/oval (malignant) glands (approx. 5 cells across) interspersed with larger (benign) glands that are 2-3 times larger. | **"Infiltrative": small round/oval (malignant) glands (approx. 5 cells across) interspersed with larger (benign) glands that are 2-3 times larger. | ||
===High power features=== | ====High power features==== | ||
* | *Nuclear changes. | ||
**Hyperchromatic nuclei (like in HGPIN). | **Hyperchromatic nuclei (like in HGPIN). | ||
**Nuclear enlargement. | **Nuclear enlargement, mild (10%?). | ||
***Difficult to appreciate (if cancer isn't side-by-side with normal prostate). | ***Difficult to appreciate (if cancer isn't side-by-side with normal prostate). | ||
***Difficult to see | ***Difficult/impossible to see at low power. | ||
* | *"Large" nucleoli. | ||
**May be difficult to see - especially if light intensity is low. | **Visible on intermediate and high power (100x / 200x magnification). | ||
***One should not use 400x to look for nucleoli (it is a waste of time + you risk | ***May be difficult to see - especially if light intensity is low or the staining is of poor quality. | ||
** | ***One should not use 400x to look for nucleoli (it is a waste of time + you risk over-calling something benign). | ||
**"Large" is rarely precisely quantified; 3 micrometres has been suggested as "large" based on one study.<ref name=pmid1688728>{{Cite journal | last1 = Kelemen | first1 = PR. | last2 = Buschmann | first2 = RJ. | last3 = Weisz-Carrington | first3 = P. | title = Nucleolar prominence as a diagnostic variable in prostatic carcinoma. | journal = Cancer | volume = 65 | issue = 4 | pages = 1017-20 | month = Feb | year = 1990 | doi = | PMID = 1688728 }}</ref> | |||
***Three micrometres is a little more than 1/3 of [[RBC]] diameter. | |||
*Loss of basal cells - diagnostic feature. | *Loss of basal cells - diagnostic feature. | ||
**Like in breast pathology (where one looks for loss of myoepithelial cells) - this may be difficult to see. | **Like in [[breast pathology]] (where one looks for loss of myoepithelial cells) - this may be difficult to see. | ||
Notes: | Notes: | ||
*Mitoses are not a common feature - | *Mitoses are not a common feature. | ||
**If you find them the lesion is probably high-grade. | |||
**Generally, it isn't worth looking for them. | |||
===Mimics=== | ===Mimics=== | ||
| Line 120: | Line 189: | ||
| many small glands, lack nuclear size variation, basal layer present | | many small glands, lack nuclear size variation, basal layer present | ||
| nucleoli may be present; may need to do p63 or 34betaE12 to find basal layer | | nucleoli may be present; may need to do p63 or 34betaE12 to find basal layer | ||
| [ | | [[Image:Adenosis of prostate_--_intermed_mag.jpg|thumb|150px|center| Adenosis of prostate. (WC)]] | ||
|- | |- | ||
| Sclerosing adenosis | | Sclerosing adenosis | ||
| gradual transition between normal & small gland (NOT two populations), fibrosis | | gradual transition between normal & small gland (NOT two populations), fibrosis | ||
| many small glands, lack nuclear size variation, basal layer present | | many small glands, lack nuclear size variation, basal layer present | ||
| analogous to sclerosing adenosis of breast | | analogous to [[sclerosing adenosis of the breast]]{{fact}} | ||
| [http://webpathology.com/image.asp?case=21&n= | | [http://webpathology.com/image.asp?case=21&n=40 Sclerosing adenosis (webpathology.com)] | ||
|- | |- | ||
| [[atrophy of the prostate|Atrophy]] | | [[atrophy of the prostate|Atrophy]] | ||
| Line 132: | Line 201: | ||
| nuclear hyperchromasia, scant cytoplasm | | nuclear hyperchromasia, scant cytoplasm | ||
| may appear right beside non-atrophic tissue | | may appear right beside non-atrophic tissue | ||
| [ | | [[Image:Atrophic_prostatic_glands_--_high_mag.jpg|thumb|150px|center| Prostatic atrophy. (WC)]] | ||
|- | |- | ||
| [[Basal cell hyperplasia of the prostate|Basal cell hyperplasia]] | | [[Basal cell hyperplasia of the prostate|Basal cell hyperplasia]] | ||
| Line 138: | Line 207: | ||
| abundant epithelial cells; nucleoli in pale ('blue') nuclei of basal cells, glandular cell nuclei darker ('purple') | | abundant epithelial cells; nucleoli in pale ('blue') nuclei of basal cells, glandular cell nuclei darker ('purple') | ||
| vaguely similar to epithelial hyperplasia of usual type (EHUT) in breast | | vaguely similar to epithelial hyperplasia of usual type (EHUT) in breast | ||
| [ | | [[Image:Basal_cell_hyperplasia_of_prostate_-_high_mag.jpg|thumb|150px|center| Prostatic BCH. (WC)]] | ||
|- | |- | ||
| [[Bulbourethral gland]] | | [[Bulbourethral gland]] | ||
| Line 144: | Line 213: | ||
| clear cytoplasm | | clear cytoplasm | ||
| apex of prostate | | apex of prostate | ||
| [ | | [[Image:Bulbourethral gland -- very high mag.jpg |thumb|150px|center| Bulbourethral gland. (WC)]] | ||
|- | |- | ||
| [[Seminal vesicles]] / ejaculatory ducts | | [[Seminal vesicles]] / ejaculatory ducts | ||
| Line 150: | Line 219: | ||
| fern-like arrangement of epithelium (low power), nucleoli, surrounded by muscle, +/- nuclear inclusions | | fern-like arrangement of epithelium (low power), nucleoli, surrounded by muscle, +/- nuclear inclusions | ||
| involvement by cancer changes staging, lipofuscin may be present in prostate, often has marked nuc. size var.; location: usu. base of prostate | | involvement by cancer changes staging, lipofuscin may be present in prostate, often has marked nuc. size var.; location: usu. base of prostate | ||
| [ | | [[Image:Seminal_vesicle_high_mag.jpg |thumb|150px|center|Seminal vesicles. (WC)]] | ||
|- | |- | ||
| Radiation effect | | [[Radiation effect]] | ||
| marked nuclear size variation | | marked nuclear size variation | ||
| increased stroma (fibrosis), lack nucleoli ??? | | increased stroma (fibrosis), lack nucleoli ??? | ||
| history of Rx; uniform nuc. size with Hx of Rx should raise susp. of cancer | | history of Rx; uniform nuc. size with Hx of Rx should raise susp. of [[postradiation prostatic carcinoma|postradiation cancer]] | ||
| [ | | [[Image:Prostate_with_radiation_changes_--_high_mag.jpg|thumb|150px|center|Radiation change. (WC)]] | ||
|- | |- | ||
| Prostatitis | | Prostatitis | ||
| Line 162: | Line 231: | ||
| no nuclear atypia, normal gland arch. | | no nuclear atypia, normal gland arch. | ||
| clinical mimic of cancer (elevated PSA); usu. not a problem for the pathologist | | clinical mimic of cancer (elevated PSA); usu. not a problem for the pathologist | ||
| [ | | [[Image:Inflammation_of_prostate.jpg|thumb|150px|center| Prostatic inflammation. (WC)]] | ||
|- | |- | ||
| [[Vasitis nodosa]] | | [[Vasitis nodosa]] | ||
| Line 168: | Line 237: | ||
| small tubules, nucleoli common, mild atypia, may "invade" vessels, track along nerves | | small tubules, nucleoli common, mild atypia, may "invade" vessels, track along nerves | ||
| mimics metastatic prostate carcinoma, IHC stains: PSA-, PSAP- | | mimics metastatic prostate carcinoma, IHC stains: PSA-, PSAP- | ||
| [ | | [[Image:Vasitis nodosa -11- intermed mag.jpg|thumb|150px|center| VN. (WC)]] | ||
|} | |} | ||
Memory device: '''AAABBRS''' = atrophy, adenosis, adenosis (sclerosing), basal cell hyperplasia, bulbourethral gland, radiation, seminal vesicles. | Memory device: '''AAABBRS''' = atrophy, adenosis, adenosis (sclerosing), basal cell hyperplasia, bulbourethral gland, radiation, seminal vesicles. | ||
=== | ===Situations where prostate adenocarcinoma may be missed=== | ||
Key reasons for false negative prostate samples<ref>{{cite journal |authors=Yang C, Humphrey PA |title=False-Negative Histopathologic Diagnosis of Prostatic Adenocarcinoma |journal=Arch. Pathol. Lab. Med. |volume=144 |issue=3 |pages=326–334 |date=March 2020 |pmid=31729886 |doi=10.5858/arpa.2019-0456-RA |url=}}</ref>: | |||
*Tissue artefacts (try levels and/or IHC): | |||
* | **Crush artefact | ||
** | **Thick sections | ||
* | **Aberrant H&E staining | ||
** | **Freezing artefact | ||
* | **Cautery | ||
*Minimal adenocarcinoma (less than 1mm long or involving less than 5% of a core biopsy): | |||
* | *Prostatic adenocarcinoma variants that mimic benign: | ||
**[[Atrophic prostate carcinoma]] | |||
**[[Pseudohyperplastic adenocarcinoma]] | |||
**[[Foamy gland adenocarcinoma]] | |||
* | **[[PIN-like adenocarcinoma]] | ||
* | **Microcystic adenocarcinoma | ||
*Single cells of Gleason 5 adenocarcinoma (missed or mistaken for lymphocytes; try IHC for cytokeratins, prostatic and/or hematologic markers) | |||
*Treatment effect (check clinical information and look for treatment effect in benign glands) | |||
* | |||
** | |||
=== | ===Prostate cancer grading=== | ||
{{Main|Prostate cancer grading}} | |||
It covers the ''Gleason grading system'' and the (new) ''prognostic grade groupings''. | |||
===Staging parameters, margins and more=== | |||
===Staging parameters and | |||
====Surgical margins==== | ====Surgical margins==== | ||
{{Main|Surgical margins}} | {{Main|Surgical margins}} | ||
*Positive is ''tumour touching ink''.<ref name=pmid22578729>{{Cite journal | last1 = Lu | first1 = J. | last2 = Wirth | first2 = GJ. | last3 = Wu | first3 = S. | last4 = Chen | first4 = J. | last5 = Dahl | first5 = DM. | last6 = Olumi | first6 = AF. | last7 = Young | first7 = RH. | last8 = McDougal | first8 = WS. | last9 = Wu | first9 = CL. | title = A close surgical margin after radical prostatectomy is an independent predictor of recurrence. | journal = J Urol | volume = 188 | issue = 1 | pages = 91-7 | month = Jul | year = 2012 | doi = 10.1016/j.juro.2012.02.2565 | PMID = 22578729 }}</ref> | *Positive is ''tumour touching [[ink]]''.† <ref name=pmid22578729>{{Cite journal | last1 = Lu | first1 = J. | last2 = Wirth | first2 = GJ. | last3 = Wu | first3 = S. | last4 = Chen | first4 = J. | last5 = Dahl | first5 = DM. | last6 = Olumi | first6 = AF. | last7 = Young | first7 = RH. | last8 = McDougal | first8 = WS. | last9 = Wu | first9 = CL. | title = A close surgical margin after radical prostatectomy is an independent predictor of recurrence. | journal = J Urol | volume = 188 | issue = 1 | pages = 91-7 | month = Jul | year = 2012 | doi = 10.1016/j.juro.2012.02.2565 | PMID = 22578729 }}</ref> | ||
**"Close" margins have an | **"Close" margins (<0.1 mm) have an increased recurrence risk.<ref name=pmid22578729/> | ||
Notes: | Notes: | ||
| Line 338: | Line 273: | ||
**It is possible to have EPE without a positive margin. | **It is possible to have EPE without a positive margin. | ||
**It is possible to have a positive margin without EPE. | **It is possible to have a positive margin without EPE. | ||
* † Epstein says not touching may be enough, as tumour close to the margin is damaged from the surgery.<ref>URL: [http://urology.jhu.edu/newsletter/prostate_cancer410.php http://urology.jhu.edu/newsletter/prostate_cancer410.php]. Accessed on: 26 March 2013.</ref> | |||
=====Rates and implication===== | =====Rates and implication===== | ||
Positivity rate varies substantially (13-44%): | Positivity rate varies substantially (13-44%): | ||
*Norway: 26% -- strong dependence on surgeon volume (18% high case load vs. 44% low case load).<ref name=pmid22860630>{{Cite journal | last1 = Steinsvik | first1 = EA. | last2 = Axcrona | first2 = K. | last3 = Angelsen | first3 = A. | last4 = Beisland | first4 = C. | last5 = Dahl | first5 = A. | last6 = Eri | first6 = LM. | last7 = Haug | first7 = ES. | last8 = Svindland | first8 = A. | last9 = Fosså | first9 = S. | title = Does a surgeon's annual radical prostatectomy volume predict the risk of positive surgical margins and urinary incontinence at one-year follow-up? - Findings from a prospective national study. | journal = Scand J Urol Nephrol | volume = | issue = | pages = | month = Aug | year = 2012 | doi = 10.3109/00365599.2012.707684 | PMID = 22860630 }}</ref> | *Norway: 26% -- strong dependence on surgeon volume (18% high case load vs. 44% low case load).<ref name=pmid22860630>{{Cite journal | last1 = Steinsvik | first1 = EA. | last2 = Axcrona | first2 = K. | last3 = Angelsen | first3 = A. | last4 = Beisland | first4 = C. | last5 = Dahl | first5 = A. | last6 = Eri | first6 = LM. | last7 = Haug | first7 = ES. | last8 = Svindland | first8 = A. | last9 = Fosså | first9 = S. | title = Does a surgeon's annual radical prostatectomy volume predict the risk of positive surgical margins and urinary incontinence at one-year follow-up? - Findings from a prospective national study. | journal = Scand J Urol Nephrol | volume = | issue = | pages = | month = Aug | year = 2012 | doi = 10.3109/00365599.2012.707684 | PMID = 22860630 }}</ref> | ||
*France: 13-17%.<ref name=pmid22860572>{{Cite journal | last1 = Koutlidis | first1 = N. | last2 = Mourey | first2 = E. | last3 = Champigneulle | first3 = J. | last4 = Mangin | first4 = P. | last5 = Cormier | first5 = L. | title = Robot-assisted or pure laparoscopic nerve-sparing radical prostatectomy: What is the optimal procedure for the surgical margins? A single center experience. | journal = Int J Urol | volume = | issue = | pages = | month = Jul | year = 2012 | doi = 10.1111/j.1442-2042.2012.03102.x | PMID = 22860572 }}</ref> | *France: 13-17% -- PSA and prostate size predictors of positivity.<ref name=pmid22860572>{{Cite journal | last1 = Koutlidis | first1 = N. | last2 = Mourey | first2 = E. | last3 = Champigneulle | first3 = J. | last4 = Mangin | first4 = P. | last5 = Cormier | first5 = L. | title = Robot-assisted or pure laparoscopic nerve-sparing radical prostatectomy: What is the optimal procedure for the surgical margins? A single center experience. | journal = Int J Urol | volume = | issue = | pages = | month = Jul | year = 2012 | doi = 10.1111/j.1442-2042.2012.03102.x | PMID = 22860572 }}</ref> | ||
Note: | Note: | ||
* | *Stage and grade (Gleason score) seem to have less impact than surgeons volume on margin positivity rate.<ref name=pmid22860630/> | ||
The impact of positive margins: | The impact of positive margins: | ||
*Significant modest negative affect on long-term outcome in node negative cancers (pT2-4 pN0).<ref name=pmid22901983>{{Cite journal | last1 = Mauermann | first1 = J. | last2 = Fradet | first2 = V. | last3 = Lacombe | first3 = L. | last4 = Dujardin | first4 = T. | last5 = Tiguert | first5 = R. | last6 = Tetu | first6 = B. | last7 = Fradet | first7 = Y. | title = The Impact of Solitary and Multiple Positive Surgical Margins on Hard Clinical End Points in 1712 Adjuvant Treatment-Naive pT2-4 N0 Radical Prostatectomy Patients. | journal = Eur Urol | volume = | issue = | pages = | month = Aug | year = 2012 | doi = 10.1016/j.eururo.2012.08.002 | PMID = 22901983 }}</ref> | *Significant modest negative affect on long-term outcome in node negative cancers (pT2-4 pN0).<ref name=pmid22901983>{{Cite journal | last1 = Mauermann | first1 = J. | last2 = Fradet | first2 = V. | last3 = Lacombe | first3 = L. | last4 = Dujardin | first4 = T. | last5 = Tiguert | first5 = R. | last6 = Tetu | first6 = B. | last7 = Fradet | first7 = Y. | title = The Impact of Solitary and Multiple Positive Surgical Margins on Hard Clinical End Points in 1712 Adjuvant Treatment-Naive pT2-4 N0 Radical Prostatectomy Patients. | journal = Eur Urol | volume = | issue = | pages = | month = Aug | year = 2012 | doi = 10.1016/j.eururo.2012.08.002 | PMID = 22901983 }}</ref> | ||
*Weaker impact than stage and Gleason score.<ref>{{Cite journal | last1 = Chalfin | first1 = HJ. | last2 = Dinizo | first2 = M. | last3 = Trock | first3 = BJ. | last4 = Feng | first4 = Z. | last5 = Partin | first5 = AW. | last6 = Walsh | first6 = PC. | last7 = Humphreys | first7 = E. | last8 = Han | first8 = M. | title = Impact of surgical margin status on prostate-cancer-specific mortality. | journal = BJU Int | volume = | issue = | pages = | month = Jul | year = 2012 | doi = 10.1111/j.1464-410X.2012.11371.x | PMID = 22788795 }}</ref> | *Weaker impact than stage and Gleason score.<ref>{{Cite journal | last1 = Chalfin | first1 = HJ. | last2 = Dinizo | first2 = M. | last3 = Trock | first3 = BJ. | last4 = Feng | first4 = Z. | last5 = Partin | first5 = AW. | last6 = Walsh | first6 = PC. | last7 = Humphreys | first7 = E. | last8 = Han | first8 = M. | title = Impact of surgical margin status on prostate-cancer-specific mortality. | journal = BJU Int | volume = | issue = | pages = | month = Jul | year = 2012 | doi = 10.1111/j.1464-410X.2012.11371.x | PMID = 22788795 }}</ref> | ||
*Bladder neck margin positivity may change the T-stage - see below. | |||
=====Bladder neck margin===== | |||
{{Main|Bladder neck invasion}} | |||
:[[AKA]] ''invasion of the bladder neck''.<ref name=pmid19914651/> | |||
*Bladder neck margin positivity typically is '''pT3a'''.<ref name=pmid23225909>{{Cite journal | last1 = Chung | first1 = MS. | last2 = Lee | first2 = SH. | last3 = Lee | first3 = DH. | last4 = Chung | first4 = BH. | title = Evaluation of the 7th American Joint Committee on cancer TNM staging system for prostate cancer in point of classification of bladder neck invasion. | journal = Jpn J Clin Oncol | volume = 43 | issue = 2 | pages = 184-8 | month = Feb | year = 2013 | doi = 10.1093/jjco/hys196 | PMID = 23225909 }}</ref> | |||
*Seen in approximately 1% of prostatectomies.<ref name=pmid19914651>{{Cite journal | last1 = Pierorazio | first1 = PM. | last2 = Epstein | first2 = JI. | last3 = Humphreys | first3 = E. | last4 = Han | first4 = M. | last5 = Walsh | first5 = PC. | last6 = Partin | first6 = AW. | title = The significance of a positive bladder neck margin after radical prostatectomy: the American Joint Committee on Cancer Pathological Stage T4 designation is not warranted. | journal = J Urol | volume = 183 | issue = 1 | pages = 151-7 | month = Jan | year = 2010 | doi = 10.1016/j.juro.2009.08.138 | PMID = 19914651 }}</ref> | |||
====Extraprostatic extension==== | ====Extraprostatic extension==== | ||
:Abbreviated ''EPE''. | :Abbreviated ''EPE''. | ||
{{Main|Prostate cancer staging#Extraprostatic extension}} | |||
==== | ====Seminal vesicle invasion==== | ||
:Abbreviated ''SVI''. | |||
{{Main|Prostate cancer staging#Seminal vesicle invasion}} | |||
===== | ====Perineural invasion==== | ||
{{Main|Perineural invasion}} | |||
*''Not'' a staging parameter. | |||
*Seen in approximately 20% of core biopsies.<ref name=pmid16096404>{{Cite journal | last1 = Ali | first1 = TZ. | last2 = Epstein | first2 = JI. | title = Perineural involvement by benign prostatic glands on needle biopsy. | journal = Am J Surg Pathol | volume = 29 | issue = 9 | pages = 1159-63 | month = Sep | year = 2005 | doi = | PMID = 16096404 }}</ref> | |||
*Complete wrapping of a nerve by epithelium is considered pathognomonic for cancer.<ref name=pmid10435561>{{Cite journal | last1 = Baisden | first1 = BL. | last2 = Kahane | first2 = H. | last3 = Epstein | first3 = JI. | title = Perineural invasion, mucinous fibroplasia, and glomerulations: diagnostic features of limited cancer on prostate needle biopsy. | journal = Am J Surg Pathol | volume = 23 | issue = 8 | pages = 918-24 | month = Aug | year = 1999 | doi = | PMID = 10435561 }}</ref><ref name=pmid16096404/> | |||
Note: | Note: | ||
* | *Occasionally, benign glands are found perineural.<ref name=pmid16096404/> | ||
**These should ''not'' completely wrap around the nerve and should be cytologically benign. | |||
===== | ==IHC== | ||
===General recommendations=== | |||
ISUP consensus statement:<ref name=pmid25025364>{{cite journal |author=Amin MB, Epstein JI, Ulbright TM, ''et al.'' |title=Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the international society of urological pathology consensus conference |journal=Am. J. Surg. Pathol. |volume=38 |issue=8 |pages=1017–22 |year=2014 |month=August |pmid=25025364 |doi=10.1097/PAS.0000000000000254 |url=}}</ref> | |||
*Should ''not'' be used if cancer is obvious. | |||
*Should ''not'' be used if it isn't going change the clinical management. | |||
==== | ===Prostate markers=== | ||
*[[PSA]] (prostate specific antigen) +ve. | |||
* | *[[PSAP]] (prostatic specific acid phosphatase) +ve. † | ||
*P501S +ve. ‡ | |||
*[[NKX3.1]] +ve. ‡ | |||
* | |||
Notes: | Notes: | ||
* | *† PSAP may be positive in hindgut [[neuroendocrine tumour]]s.<ref name=pmid>{{Cite journal | last1 = Azumi | first1 = N. | last2 = Traweek | first2 = ST. | last3 = Battifora | first3 = H. | title = Prostatic acid phosphatase in carcinoid tumors. Immunohistochemical and immunoblot studies. | journal = Am J Surg Pathol | volume = 15 | issue = 8 | pages = 785-90 | month = Aug | year = 1991 | doi = | PMID = 1712549 }}</ref> | ||
*‡ P501S and NKX3.1 are considered second line markers.<ref name=pmid25025364/> | |||
*Prostate carcinoma is typically CK7 -ve and CK20 -ve; however, in high [[Gleason score]] cancers focal positivity of these markers can be seen.<ref name=pmid11888088>{{Cite journal | last1 = Goldstein | first1 = NS. | title = Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. | journal = Am J Clin Pathol | volume = 117 | issue = 3 | pages = 471-7 | month = Mar | year = 2002 | doi = 10.1309/G6PR-Y774-X738-FG2K | PMID = 11888088 }}</ref> | |||
**CK7: >25-50% staining seen in ~5% of cases. | |||
***>50% staining with CK7 is not report. | |||
**CK20: >25-50% staining seen in ~10% of cases. | |||
***>50% staining with CK20 is not reported. | |||
===Benign prostate versus neoplastic prostate=== | ===Benign prostate versus neoplastic prostate=== | ||
*AMACR +ve. | *AMACR +ve. | ||
*p63 -ve. | *p63 -ve. | ||
*HMWCK (34betaE12) -ve. | *HMWCK (34betaE12) -ve. | ||
| Line 400: | Line 345: | ||
****A. '''CA'''ncer of the '''P'''rostate. | ****A. '''CA'''ncer of the '''P'''rostate. | ||
Other IHC stains: | |||
* | *AR +ve -- in prostate confined cancer. | ||
* | **Usually -ve in lymph node +ve disease.<ref name=pmid20878946>{{Cite journal | last1 = Fleischmann | first1 = A. | last2 = Rocha | first2 = C. | last3 = Schobinger | first3 = S. | last4 = Seiler | first4 = R. | last5 = Wiese | first5 = B. | last6 = Thalmann | first6 = GN. | title = Androgen receptors are differentially expressed in Gleason patterns of prostate cancer and down-regulated in matched lymph node metastases. | journal = Prostate | volume = 71 | issue = 5 | pages = 453-60 | month = Apr | year = 2011 | doi = 10.1002/pros.21259 | PMID = 20878946 }}</ref> | ||
Note: | Note: | ||
*AMACR not useful | *Bcl-2 marks basal cells in prostate cancer.<ref name=pmid20189848>{{Cite journal | last1 = Boran | first1 = C. | last2 = Kandirali | first2 = E. | last3 = Yilmaz | first3 = F. | last4 = Serin | first4 = E. | last5 = Akyol | first5 = M. | title = Reliability of the 34βE12, keratin 5/6, p63, bcl-2, and AMACR in the diagnosis of prostate carcinoma. | journal = Urol Oncol | volume = 29 | issue = 6 | pages = 614-23 | month = | year = | doi = 10.1016/j.urolonc.2009.11.013 | PMID = 20189848 }}</ref> | ||
====Prostate carcinoma versus urothelial carcinoma==== | |||
The ISUP panel recommends:<ref name=pmid25025364>{{cite journal |author=Amin MB, Epstein JI, Ulbright TM, ''et al.'' |title=Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the international society of urological pathology consensus conference |journal=Am. J. Surg. Pathol. |volume=38 |issue=8 |pages=1017–22 |year=2014 |month=August |pmid=25025364 |doi=10.1097/PAS.0000000000000254 |url=}}</ref> | |||
*PSA +ve (-ve in UCC). | |||
*GATA3 -ve (+ve in UCC). | |||
Another panel - if GATA3 isn't available: | |||
*Prostate: PSA +ve, p63 -ve, HWMCK -ve. | |||
*Urothelial: p63 +ve, HWMCK +ve, PSA -ve. | |||
Notes: | |||
*AMACR not useful; it is positive in ~50% of [[UCC]].<ref name=pmid16315020>{{Cite journal | last1 = Langner | first1 = C. | last2 = Rupar | first2 = G. | last3 = Leibl | first3 = S. | last4 = Hutterer | first4 = G. | last5 = Chromecki | first5 = T. | last6 = Hoefler | first6 = G. | last7 = Rehak | first7 = P. | last8 = Zigeuner | first8 = R. | title = Alpha-methylacyl-CoA racemase (AMACR/P504S) protein expression in urothelial carcinoma of the upper urinary tract correlates with tumour progression. | journal = Virchows Arch | volume = 448 | issue = 3 | pages = 325-30 | month = Mar | year = 2006 | doi = 10.1007/s00428-005-0129-6 | PMID = 16315020 }}</ref> | |||
*CK7 and CK20 are typically negative in prostate carcinoma, and classically positive in urothelial carcinoma. | |||
*CK34betaE12 may be positive in prostate cancer; 43% of cases in one small series of cases with lymph node metastases.<ref name=pmid9024071>{{Cite journal | last1 = Googe | first1 = PB. | last2 = McGinley | first2 = KM. | last3 = Fitzgibbon | first3 = JF. | title = Anticytokeratin antibody 34 beta E12 staining in prostate carcinoma. | journal = Am J Clin Pathol | volume = 107 | issue = 2 | pages = 219-23 | month = Feb | year = 1997 | doi = | PMID = 9024071 }}</ref> | |||
===Rate of utilization=== | |||
*Dependent on practise setting. | |||
**One tertiary academic institution uses it on ~ 40% of cases.<ref>{{Cite journal | last1 = Watson | first1 = K. | last2 = Wang | first2 = C. | last3 = Yilmaz | first3 = A. | last4 = Bismar | first4 = TA. | last5 = Trpkov | first5 = K. | title = Use of immunohistochemistry in routine workup of prostate needle biopsies: a tertiary academic institution experience. | journal = Arch Pathol Lab Med | volume = 137 | issue = 4 | pages = 541-5 | month = Apr | year = 2013 | doi = 10.5858/arpa.2012-0145-OA | PMID = 23273390 }}</ref> | |||
==Molecular changes in prostate cancer== | ==Molecular changes in prostate cancer== | ||
| Line 416: | Line 379: | ||
==Sign out== | ==Sign out== | ||
===Prostatectomy specimens=== | ===Prostatectomy specimens=== | ||
*A prostatectomy that appears to be negative should be worked-up. This is discuss in the ''[[negative prostatectomy]]'' article. | |||
*[http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=/portlets/contentViewer/show&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=committees/cancer/cancer_protocols/protocols_index.html&_pageLabel=cntvwr CAP checklist]. | |||
<pre> | |||
A. LYMPH NODES, RIGHT PELVIC, EXCISION: | |||
- ONE LYMPH NODE NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 1 ). | |||
B. LYMPH NODES, LEFT PELVIC, EXCISION: | |||
- ONE LYMPH NODE NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 1 ). | |||
C. PROSTATE GLAND AND SEMINAL VESICLES, RADICAL PROSTATECTOMY: | |||
- ADENOCARCINOMA, GLEASON SCORE 7/10 (3+4), pT2c pN0. | |||
-- SURGICAL MARGINS NEGATIVE. | |||
-- PLEASE SEE TUMOUR SUMMARY. | |||
</pre> | |||
===Transurethral resection of prostate=== | |||
<pre> | |||
Prostate Tissue, Transurethral Resection of Prostate (TURP): | |||
- ADENOCARCINOMA, Gleason score 6/10 (3+3); | |||
-- Approximately 2% of tissue involved; | |||
-- Please see tumour summary. | |||
Comment: | |||
The World Health Organization (WHO) grade is: 1 out of 5. | |||
</pre> | |||
<pre> | |||
Prostate Tissue, Transurethral Resection of Prostate (TURP): | |||
- ADENOCARCINOMA, Gleason score 7/10 (3+4); | |||
-- Approximately 4% of tissue involved; | |||
-- Please see tumour summary. | |||
- Benign inflamed urothelium. | |||
Comment: | |||
The World Health Organization (WHO) grade is: 2 out of 5. Gleason pattern 3 represents 90% of the tumour, and Gleason pattern 4 represents 10% of the tumour. | |||
</pre> | |||
====Block letters==== | |||
<pre> | |||
PROSTATE TISSUE, TRANSURETHRAL RESECTION OF PROSTATE (TURP): | |||
- ADENOCARCINOMA, GLEASON SCORE 7/10 (3+4); | |||
- APPROXIMATELY 5% OF TISSUE INVOLVED; | |||
- PLEASE SEE TUMOUR SUMMARY. | |||
TUMOUR SUMMARY - TRANSURETHRAL RESECTION OF PROSTATE (TURP). | |||
PROCEDURE: TRANSURETHRAL PROSTATIC RESECTION. | |||
SPECIMEN SIZE: WEIGHT: 10 GRAMS. | |||
HISTOLOGIC TYPE: ADENOCARCINOMA (ACINAR, NOT OTHERWISE SPECIFIED). | |||
HISTOLOGIC GRADING: | |||
PRIMARY PATTERN: 3. | |||
SECONDARY PATTERN: 4 (40% OF TUMOUR). | |||
TOTAL GLEASON SCORE: 7 (3+4). | |||
TUMOUR QUANTITATION - PERCENTAGE OF PROSTATIC TISSUE INVOLVED BY TUMOUR: 80 %. | |||
PERIPROSTATIC FAT INVASION: NOT IDENTIFIED. | |||
SEMINAL VESICLE INVASION: NOT IDENTIFIED. | |||
LYMPH-VASCULAR INVASION: NOT IDENTIFIED. | |||
PERINEURAL INVASION: NOT IDENTIFIED. | |||
ADDITIONAL PATHOLOGIC FINDINGS: | |||
HIGH-GRADE PROSTATIC INTRAEPITHELIAL NEOPLASIA (HGPIN). | |||
NODULAR PROSTATIC HYPERPLASIA. | |||
CHRONIC INFLAMMATION. | |||
</pre> | |||
<pre> | |||
PROSTATE TISSUE, TRANSURETHRAL RESECTION OF THE PROSTATE (TURP): | |||
- ADENOCARCINOMA, GLEASON SCORE 6/10 (3+3); | |||
- APPROXIMATELY 5% OF TISSUE INVOLVED; | |||
- PLEASE SEE TUMOUR SUMMARY. | |||
</pre> | |||
<pre> | |||
Prostate Tissue, Transurethral Resection of Prostate (TURP): | |||
- Adenocarcinoma, Gleason score 7 (3+4) | |||
- Approximately 3% of sampled tissue involved | |||
- Please see tumour summary | |||
Tumour summary: | |||
Procedure: Transurethral resection of prostate | |||
Specimen weight: 11.7 g | |||
Histologic type: Adenocarcinoma, acinar type | |||
Histologic grade: | |||
Primary pattern: 3 | |||
Secondary pattern: 4 (<15% of tumor) | |||
Total Gleason score: 7/10 (3+4) | |||
Tumor volume: 3% of tissue | |||
Periprostate fat invasion: Periprostatic fat not identified | |||
Seminal vesicle invasion: Seminal vesicle not identified | |||
Lymphovascular inasion: Not identified | |||
Perineural invasion: Not identified | |||
Additional findings: | |||
Glandular and stromal hyperplasia | |||
Mild chronic inflammation | |||
</pre> | |||
===Biopsy specimens=== | ===Biopsy specimens=== | ||
| Line 430: | Line 496: | ||
Notes: | Notes: | ||
*‡ "Percent area involved" may seem like an odd thing to request 'cause it is sampling dependent, i.e. if the radiologist sticks the biopsy needle deeper into the lesion more of the core is positive, but urologists think it is important -- more important than perineural invasion.<ref name=pmid15223967>{{cite journal |author=Rubin MA, Bismar TA, Curtis S, Montie JE |title=Prostate needle biopsy reporting: how are the surgical members of the Society of Urologic Oncology using pathology reports to guide treatment of prostate cancer patients? |journal=Am. J. Surg. Pathol. |volume=28 |issue=7 |pages=946–52 |year=2004 |month=July |pmid=15223967 |doi= |url=}}</ref> | *‡ "Percent area involved" may seem like an odd thing to request 'cause it is sampling dependent, i.e. if the radiologist sticks the biopsy needle deeper into the lesion more of the core is positive, but urologists think it is important -- more important than perineural invasion.<ref name=pmid15223967>{{cite journal |author=Rubin MA, Bismar TA, Curtis S, Montie JE |title=Prostate needle biopsy reporting: how are the surgical members of the Society of Urologic Oncology using pathology reports to guide treatment of prostate cancer patients? |journal=Am. J. Surg. Pathol. |volume=28 |issue=7 |pages=946–52 |year=2004 |month=July |pmid=15223967 |doi= |url=}}</ref> | ||
**There is disagreement on how one should measure patchy cancer (cancer when there is interspersed normal). Epstein believes one should include the interspersed benign if the cancer is patchy, as | **There is disagreement on how one should measure patchy cancer (cancer when there is interspersed normal). Epstein believes one should include the interspersed benign if the cancer is patchy, as the groupings of tumour likely join out of the plane of section.<ref name=pmid21788055>{{Cite journal | last1 = Epstein | first1 = JI. | title = Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens. | journal = J Urol | volume = 186 | issue = 3 | pages = 790-7 | month = Sep | year = 2011 | doi = 10.1016/j.juro.2011.02.2695 | PMID = 21788055 }}</ref> | ||
**A review by Epstein on the topic of tumour volume suggests it does not have predictive value in multivariante analyses.<ref name=pmid21788055/> | **A review by Epstein on the topic of tumour volume suggests it does not have predictive value in multivariante analyses.<ref name=pmid21788055/> | ||
**The biopsy tumour volume is a predictor of Gleason score upgrading on prostatectomy.<ref name=pmid22688447>{{Cite journal | last1 = Fu | first1 = Q. | last2 = Moul | first2 = JW. | last3 = Bañez | first3 = LL. | last4 = Sun | first4 = L. | last5 = Mouraviev | first5 = V. | last6 = Xie | first6 = D. | last7 = Polascik | first7 = TJ. | title = Association between percentage of tumor involvement and Gleason score upgrading in low-risk prostate cancer. | journal = Med Oncol | volume = 29 | issue = 5 | pages = 3339-44 | month = Dec | year = 2012 | doi = 10.1007/s12032-012-0270-4 | PMID = 22688447 }}</ref> | |||
====Completely negative==== | ====Completely negative==== | ||
| Line 470: | Line 537: | ||
L. PROSTATE, LEFT MEDIAL INFERIOR, BIOPSY: | L. PROSTATE, LEFT MEDIAL INFERIOR, BIOPSY: | ||
- BENIGN PROSTATE TISSUE. | - BENIGN PROSTATE TISSUE. | ||
</pre> | |||
====Negative biopsy in surveillance==== | |||
<pre> | |||
COMMENT: | |||
The previous results are noted. The absence of cancer in this biopsy may | |||
be due to sampling. | |||
</pre> | </pre> | ||
| Line 531: | Line 605: | ||
- PERINEURAL INVASION PRESENT. | - PERINEURAL INVASION PRESENT. | ||
</pre> | </pre> | ||
=====Tumour summaries===== | |||
*These are not completely without controversy. | |||
*It should be noted that treatment is driven by the highest Gleason score.{{fact}} | |||
<pre> | <pre> | ||
| Line 553: | Line 631: | ||
</pre> | </pre> | ||
<pre> | |||
TUMOUR SUMMARY - PROSTATE CORE BIOPSIES: | |||
- HISTOLOGIC TYPE: ADENOCARCINOMA (ACINAR, NOT OTHERWISE SPECIFIED). | |||
- HIGHEST GLEASON SCORE: 8 (4+4). | |||
- SUMMARY GLEASON SCORE: 7 (4+3). | |||
- PERCENT OF TUMOUR WITH PATTERN 4: 55%. | |||
- PERCENT OF TUMOUR WITH PATTERN 5: 0%. | |||
- NUMBER OF CORES POSITIVE: 12. | |||
- TOTAL NUMBER OF CORES: 12. | |||
- TOTAL LINEAR MILLIMETERS OF NEEDLE CORE TISSUE: 178 MM. | |||
- PERCENT OF NEEDLE CORE TISSUE THAT IS TUMOUR: 80%. | |||
- PERINEURAL INVASION: PRESENT. | |||
- PERIPROSTATIC FAT INVASION: PRESENT. | |||
- LYMPHOVASCULAR INVASION: NOT IDENTIFIED. | |||
- SEMINAL VESICLE INVASION: NOT IDENTIFIED. | |||
</pre> | |||
=== | ===Seminal vesicle/ejaculatory duct invasion on biopsy=== | ||
<pre> | |||
COMMENT: | |||
The seminal vesicles and ejaculatory ducts have the same histology; thus, it is not | |||
usually possible to confidently differentiate them in a needle biopsy. | |||
SV/ED invasion was demonstrated with CK7, CK34betaE12/AMACR, PSA and p63 immunostaining. | |||
The tumour is PSA and AMACR positive. | |||
</pre> | |||
=== | =Intraductal spread of prostate cancer= | ||
==Intraductal carcinoma of the prostate== | |||
* | *[[AKA]] ''[[intraductal carcinoma]]''. | ||
* | *[[AKA]] ''intraductal prostate carcinoma''. | ||
{{Main|Intraductal carcinoma of the prostate}} | |||
=Unusual forms of prostate cancer= | =Unusual forms of prostate cancer= | ||
| Line 593: | Line 670: | ||
*[[AKA]] ''ductal adenocarcinoma of the prostate''. | *[[AKA]] ''ductal adenocarcinoma of the prostate''. | ||
*[[AKA]] ''prostatic adenocarcinoma, large duct type''. | *[[AKA]] ''prostatic adenocarcinoma, large duct type''. | ||
{{Main|Ductal adenocarcinoma of the prostate gland}} | |||
==PIN-like prostatic ductal adenocarcinoma== | ==PIN-like prostatic ductal adenocarcinoma== | ||
{{Main|High-grade prostatic intraepithelial neoplasia-like ductal adenocarcinoma of the prostate}} | |||
==Foamy gland carcinoma== | ==Foamy gland carcinoma== | ||
*[[AKA]] ''foamy gland adenocarcinoma'' | *[[AKA]] ''foamy gland adenocarcinoma''.<ref name=pmid19033862>{{Cite journal | last1 = Zhao | first1 = J. | last2 = Epstein | first2 = JI. | title = High-grade foamy gland prostatic adenocarcinoma on biopsy or transurethral resection: a morphologic study of 55 cases. | journal = Am J Surg Pathol | volume = 33 | issue = 4 | pages = 583-90 | month = Apr | year = 2009 | doi = 10.1097/PAS.0b013e31818a5c6c | PMID = 19033862 }}</ref> | ||
{{Main|Foamy gland carcinoma}} | |||
==Atrophic prostate carcinoma== | ==Atrophic prostate carcinoma== | ||
*[[AKA]] ''atrophic carcinoma''. | *[[AKA]] ''atrophic carcinoma''. | ||
{{Main|Atrophic prostate carcinoma}} | |||
==Mucinous prostate carcinoma== | ==Mucinous prostate carcinoma== | ||
{{Main|Mucinous adenocarcinoma of the prostate}} | |||
==Pseudohyperplastic prostatic adenocarcinoma== | ==Pseudohyperplastic prostatic adenocarcinoma== | ||
*[[AKA]] ''pseudohyperplastic adenocarcinoma''. | |||
* | {{Main|Pseudohyperplastic prostatic adenocarcinoma}} | ||
==Prostatic signet ring cell carcinoma== | ==Prostatic signet ring cell carcinoma== | ||
| Line 733: | Line 700: | ||
*Signet ring cells - see ''[[basics]]'' article. | *Signet ring cells - see ''[[basics]]'' article. | ||
DDx: | |||
* | *Acinar adenocarcinoma - Gleason pattern 4 with very small glands. | ||
== | ====Images==== | ||
*[[ | *[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2996149/figure/F1/ Prostatic SRCC (nih.gov)]. | ||
*[http://www.nature.com/modpathol/journal/v17/n3/fig_tab/3800052f7.html#figure-title Prostatic SRCC (nature.com)]. | |||
*[http://www.nature.com/modpathol/journal/v17/n3/fig_tab/3800052f8.html Prostatic SRCC (nature.com)]. | |||
*[http://www.webpathology.com/image.asp?case=23&n=34 Prostatic SRCC (webpathology.com)] - looks like ''acinar adenocarcinoma''. | |||
=== | ===Stains=== | ||
*Alcian blue-PAS stain +ve. | |||
* | *[[PAS stain|PAS]] -- 50% of cases +ve.<ref name=pmid21123640/> | ||
*[[Alcian blue stain|Alcian blue]] -- 44% of cases +ve.<ref name=pmid21123640/> | |||
* | |||
== | ==Sarcomatoid carcinoma of the prostate== | ||
{{Main|Sarcomatoid carcinoma of the prostate}} | |||
==Small cell carcinoma of the prostate gland== | ==Small cell carcinoma of the prostate gland== | ||
{{Main|Small cell carcinoma | {{Main|Small cell carcinoma of the prostate gland}} | ||
==Adenoid cystic/basal cell carcinoma of the prostate== | ==Adenoid cystic/basal cell carcinoma of the prostate== | ||
*Abbreviated ''ACBCC''. | *Abbreviated ''ACBCC''. | ||
{{Main|Adenoid cystic/basal cell carcinoma of the prostate}} | |||
==Postradiation prostate cancer== | |||
{{Main|Postradiation prostate cancer}} | |||
=== | =Metastatic disease and other cancers of the prostate= | ||
==Urothelial carcinoma== | |||
{{Main|Urothelial carcinoma of the urethra}} | |||
=See also= | =See also= | ||
| Line 811: | Line 739: | ||
[[Category:Genitourinary pathology]] | [[Category:Genitourinary pathology]] | ||
[[Category:Prostate carcinoma]] | |||
Latest revision as of 20:29, 24 May 2020
| Prostate carcinoma | |
|---|---|
| Diagnosis in short | |
 Prostate carcinoma. H&E stain. | |
|
| |
| LM | major criteria: abnormal architecture (increased gland density, usu. small circular glands, "infiltrative growth" pattern), basal cells lost, cytological abnormalities (nuclear enlargement, nucleoli); minor criteria: nuclear hyperchromasia, wispy blue mucin, pink amorphous secretions, intraluminal crystalloid, amphophilic cytoplasm, adjacent HGPIN, mitoses |
| LM DDx | high-grade prostatic intraepithelial neoplasia, atypical small acinar proliferation (biopsy only), prostatic atrophy, seminal vesicle, basal cell hyperplasia, others |
| IHC | PSA +ve, PSAP +ve, AMACR +ve, p63 -ve, CK34betaE12 -ve |
| Molecular | +/-BRCA1 mutation (genetic predisposition), +/-BRCA2 mutation (genetic predisposition) |
| Gross | usu. posterior aspect of the prostate - often not apparent at gross |
| Grossing notes | prostate biopsy, prostate chips, radical prostatectomy |
| Staging | prostate cancer staging |
| Site | prostate gland |
|
| |
| Signs | firm, nodular prostate on digital rectal exam |
| Symptoms | often asymptomatic |
| Prevalence | very common |
| Blood work | PSA elevated (common) |
| Radiology | hypoechoic areas, no apparent abnormality |
| Prognosis | good-to-poor (depends on grade (Gleason score) and stage) |
| Clin. DDx | prostatitis, nodular hyperplasia of the prostate |
| Treatment | observation (common for low-grade, low tumour burden), radiation or radical prostatectomy |
This article deals with prostate cancer.
The vast majority of prostate cancers are carcinomas and could be labelled prostatic carcinoma. Most prostatic carcinomas are gland forming; thus, they can be labelled prostatic adenocarcinoma or adenocarcinoma of the prostate.
Benign pathology of the prostate gland, and prostate histology and anatomy are dealt with in the prostate gland article.
Conventional prostate cancer
General
- Very common.
- Increasing incidence with age - the age in years is an approximation of the percentage of men with prostate cancer.[1][citation needed]
- Usually an indolent course - most old men die with prostate cancer not from prostate cancer.
- Risk increased with a BRCA1 or BRCA2 mutation[2] - families have a mix of breast cancer and prostate cancer.
Management
Dirty first approximation
- The management changes between Gleason score 6, 7 (3+4), 7 (4+3) and 8.
Typically, the implications are:
- Gleason 6: observation or radioactive seeds; surgery if patient wants.
- Gleason 7 with a bit of Gleason pattern 4 and a low tumour volume: it is reasonable to watch or do something. ‡
- Gleason 7 with a lot of Gleason pattern 4 or a high tumour volume: do something -- surgery or radiation therapy.
- Gleason 8+: bad cancer -- do something quickly!
Note:
- ‡ It has been said that Gleason score 7 with a bit of Gleason pattern 4 is the new Gleason score 6.
Bottom line:
- You want to be sure when you call something Gleason pattern 4.
Observational strategies
- Delay of definitive treatment (surgery or radiation).
- Common in the management of prostate cancer.
Classification:[5]
- Active surveillance (AS).
- Low risk of progression.
- May get definitive treatment later.
- Watchful waiting (WW).
- Higher risk of progression.
Note:
- There is no agreed upon set of criteria for active surveillance, and the large number of criteria out there vary significantly.[6]
Active surveillance
The Klotz criteria for active surveillance - pathologic factors only:[6][7]
Clinical criteria:
- PSA <= 10 ng/mL.[6]
- Negative DRE.
Gross
- Prostate cancer is uncommonly apparent on gross.
- Classic location: posterior aspect of the prostate.
Radiology
- Hypoechoic areas = suspicious for cancer.
Prostatectomy grossing
Cytoprostatectomy grossing
- Limited sampling of the prostate may lead to undersampling error.[14]
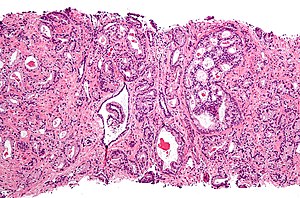
Microscopic
Criteria as a list
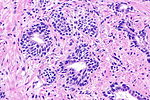
Major criteria (the ABCs of prostate pathology):[15]
- Architecture.
- Increased gland density.
- Small circular glands.
- In rare subtypes - large branching glands.
- "Infiltrative growth" pattern - malignant glands between benign ones.
- Basal cells lacking.
- Cytological abnormalities:
- Nuclear enlargement (subtle).
- Nucleoli (prominent).
Minor criteria:[15]
- Nuclear hyperchromasia.
- Wispy blue mucin.
- Image: Wispy blue mucin (nature.com) - from Epstein.[16]
- Pink amorphous secretions.
- Image: Pink amorphous secretions (nature.com) - from Epstein.[16]
- Intraluminal crystalloid.
- Image: Intraluminal crystalloid (nature.com) - from Epstein.[16]
- Amphophilic cytoplasm.
- Amphopilic is said to be bluish-red[17] -- though might also be described as blue-grey.
- Adjacent HGPIN.
- Mitoses - quite rare.
Extent/quantity criteria:
- There is no agreed upon minimum number of glands; however, one paper suggests that agreement among experts is low with 5 or less glands.[18]
- Thus, it has been suggested that six or more glands should be present to diagnose cancer.[18]
Features considered pathognomonic for prostate carcinoma by some authorities:[19][20]
- Perineural invasion.
- Must be circumferential (>95% of circumference[citation needed]).
- Glomeruloid bodies.
- Collagenous micronodules also known as mucinous fibroplasia.
Divided into high and low power
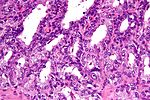
Low power features
- Architecture is the key to diagnosing low grade cancer.
- Back-to-back glands or crowding of glands -- think low grade cancer (Gleason pattern 3).
- Sharp transition between gland border and lumen.
- Loss of epithelial folding at the epithelium-gland lumen interface - "punched-out" appearance.
- Eosinophilic debris within the gland lumen (pink amorphous secretions, intraluminal crystalloid).
- Blue-tinged acellular material within the gland lumen (mucin) -- uncommon.
- "Infiltrative": small round/oval (malignant) glands (approx. 5 cells across) interspersed with larger (benign) glands that are 2-3 times larger.
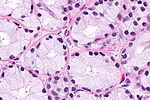
High power features
- Nuclear changes.
- Hyperchromatic nuclei (like in HGPIN).
- Nuclear enlargement, mild (10%?).
- Difficult to appreciate (if cancer isn't side-by-side with normal prostate).
- Difficult/impossible to see at low power.
- "Large" nucleoli.
- Visible on intermediate and high power (100x / 200x magnification).
- May be difficult to see - especially if light intensity is low or the staining is of poor quality.
- One should not use 400x to look for nucleoli (it is a waste of time + you risk over-calling something benign).
- "Large" is rarely precisely quantified; 3 micrometres has been suggested as "large" based on one study.[21]
- Three micrometres is a little more than 1/3 of RBC diameter.
- Visible on intermediate and high power (100x / 200x magnification).
- Loss of basal cells - diagnostic feature.
- Like in breast pathology (where one looks for loss of myoepithelial cells) - this may be difficult to see.
Notes:
- Mitoses are not a common feature.
- If you find them the lesion is probably high-grade.
- Generally, it isn't worth looking for them.
Mimics
Mimics of prostate adenocarcinoma:[22]
| Entity | Key feature | Detailed microscopic | Other | Image |
|---|---|---|---|---|
| Adenosis (AKA atypical adenomatous hyperplasia) | gradual transition between normal & small gland (NOT two populations) | many small glands, lack nuclear size variation, basal layer present | nucleoli may be present; may need to do p63 or 34betaE12 to find basal layer | |
| Sclerosing adenosis | gradual transition between normal & small gland (NOT two populations), fibrosis | many small glands, lack nuclear size variation, basal layer present | analogous to sclerosing adenosis of the breast[citation needed] | Sclerosing adenosis (webpathology.com) |
| Atrophy | sharp angulation of gland | nuclear hyperchromasia, scant cytoplasm | may appear right beside non-atrophic tissue | |
| Basal cell hyperplasia | two distinct cell populations (in epithelial component) | abundant epithelial cells; nucleoli in pale ('blue') nuclei of basal cells, glandular cell nuclei darker ('purple') | vaguely similar to epithelial hyperplasia of usual type (EHUT) in breast | |
| Bulbourethral gland | no nuclear atypia | clear cytoplasm | apex of prostate | |
| Seminal vesicles / ejaculatory ducts | lipofuscin (yellow granular material in cytoplasm), smudge cells (smeared appearance + hyperchromatic) | fern-like arrangement of epithelium (low power), nucleoli, surrounded by muscle, +/- nuclear inclusions | involvement by cancer changes staging, lipofuscin may be present in prostate, often has marked nuc. size var.; location: usu. base of prostate | |
| Radiation effect | marked nuclear size variation | increased stroma (fibrosis), lack nucleoli ??? | history of Rx; uniform nuc. size with Hx of Rx should raise susp. of postradiation cancer | |
| Prostatitis | inflammatory cells (lymphocytes, plasma cells, PMNs) | no nuclear atypia, normal gland arch. | clinical mimic of cancer (elevated PSA); usu. not a problem for the pathologist | |
| Vasitis nodosa | sperm within ducts, clinical history (usu. post-vasectomy) | small tubules, nucleoli common, mild atypia, may "invade" vessels, track along nerves | mimics metastatic prostate carcinoma, IHC stains: PSA-, PSAP- |
Memory device: AAABBRS = atrophy, adenosis, adenosis (sclerosing), basal cell hyperplasia, bulbourethral gland, radiation, seminal vesicles.
Situations where prostate adenocarcinoma may be missed
Key reasons for false negative prostate samples[23]:
- Tissue artefacts (try levels and/or IHC):
- Crush artefact
- Thick sections
- Aberrant H&E staining
- Freezing artefact
- Cautery
- Minimal adenocarcinoma (less than 1mm long or involving less than 5% of a core biopsy):
- Prostatic adenocarcinoma variants that mimic benign:
- Atrophic prostate carcinoma
- Pseudohyperplastic adenocarcinoma
- Foamy gland adenocarcinoma
- PIN-like adenocarcinoma
- Microcystic adenocarcinoma
- Single cells of Gleason 5 adenocarcinoma (missed or mistaken for lymphocytes; try IHC for cytokeratins, prostatic and/or hematologic markers)
- Treatment effect (check clinical information and look for treatment effect in benign glands)
Prostate cancer grading
It covers the Gleason grading system and the (new) prognostic grade groupings.
Staging parameters, margins and more
Surgical margins
- Positive is tumour touching ink.† [24]
- "Close" margins (<0.1 mm) have an increased recurrence risk.[24]
Notes:
- Surgical margin - where the surgeon cut.
- It is possible to have EPE without a positive margin.
- It is possible to have a positive margin without EPE.
- † Epstein says not touching may be enough, as tumour close to the margin is damaged from the surgery.[25]
Rates and implication
Positivity rate varies substantially (13-44%):
- Norway: 26% -- strong dependence on surgeon volume (18% high case load vs. 44% low case load).[26]
- France: 13-17% -- PSA and prostate size predictors of positivity.[27]
Note:
- Stage and grade (Gleason score) seem to have less impact than surgeons volume on margin positivity rate.[26]
The impact of positive margins:
- Significant modest negative affect on long-term outcome in node negative cancers (pT2-4 pN0).[28]
- Weaker impact than stage and Gleason score.[29]
- Bladder neck margin positivity may change the T-stage - see below.
Bladder neck margin
- Bladder neck margin positivity typically is pT3a.[31]
- Seen in approximately 1% of prostatectomies.[30]
Extraprostatic extension
- Abbreviated EPE.
Seminal vesicle invasion
- Abbreviated SVI.
Perineural invasion
- Not a staging parameter.
- Seen in approximately 20% of core biopsies.[32]
- Complete wrapping of a nerve by epithelium is considered pathognomonic for cancer.[20][32]
Note:
- Occasionally, benign glands are found perineural.[32]
- These should not completely wrap around the nerve and should be cytologically benign.
IHC
General recommendations
ISUP consensus statement:[33]
- Should not be used if cancer is obvious.
- Should not be used if it isn't going change the clinical management.
Prostate markers
- PSA (prostate specific antigen) +ve.
- PSAP (prostatic specific acid phosphatase) +ve. †
- P501S +ve. ‡
- NKX3.1 +ve. ‡
Notes:
- † PSAP may be positive in hindgut neuroendocrine tumours.[34]
- ‡ P501S and NKX3.1 are considered second line markers.[33]
- Prostate carcinoma is typically CK7 -ve and CK20 -ve; however, in high Gleason score cancers focal positivity of these markers can be seen.[35]
- CK7: >25-50% staining seen in ~5% of cases.
- >50% staining with CK7 is not report.
- CK20: >25-50% staining seen in ~10% of cases.
- >50% staining with CK20 is not reported.
- CK7: >25-50% staining seen in ~5% of cases.
Benign prostate versus neoplastic prostate
- AMACR +ve.
- p63 -ve.
- HMWCK (34betaE12) -ve.
Combination immunostains:
Other IHC stains:
- AR +ve -- in prostate confined cancer.
- Usually -ve in lymph node +ve disease.[39]
Note:
- Bcl-2 marks basal cells in prostate cancer.[40]
Prostate carcinoma versus urothelial carcinoma
The ISUP panel recommends:[33]
- PSA +ve (-ve in UCC).
- GATA3 -ve (+ve in UCC).
Another panel - if GATA3 isn't available:
- Prostate: PSA +ve, p63 -ve, HWMCK -ve.
- Urothelial: p63 +ve, HWMCK +ve, PSA -ve.
Notes:
- AMACR not useful; it is positive in ~50% of UCC.[41]
- CK7 and CK20 are typically negative in prostate carcinoma, and classically positive in urothelial carcinoma.
- CK34betaE12 may be positive in prostate cancer; 43% of cases in one small series of cases with lymph node metastases.[42]
Rate of utilization
- Dependent on practise setting.
- One tertiary academic institution uses it on ~ 40% of cases.[43]
Molecular changes in prostate cancer
A fusion gene between TMPRSS2 and ERG is described.[44][45]
- Both genes are on chromosome 21.
- Currently not used diagnostically.
- Fusion gene seen in approximately 50% of prostate cancer.[45]
- A subset of TMPRSS2-ERG known as 2+Edel (seen in ~7% of all prostate cancer cases) predicts poor survival.[46]
Sign out
Prostatectomy specimens
- A prostatectomy that appears to be negative should be worked-up. This is discuss in the negative prostatectomy article.
- CAP checklist.
A. LYMPH NODES, RIGHT PELVIC, EXCISION: - ONE LYMPH NODE NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 1 ). B. LYMPH NODES, LEFT PELVIC, EXCISION: - ONE LYMPH NODE NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 1 ). C. PROSTATE GLAND AND SEMINAL VESICLES, RADICAL PROSTATECTOMY: - ADENOCARCINOMA, GLEASON SCORE 7/10 (3+4), pT2c pN0. -- SURGICAL MARGINS NEGATIVE. -- PLEASE SEE TUMOUR SUMMARY.
Transurethral resection of prostate
Prostate Tissue, Transurethral Resection of Prostate (TURP): - ADENOCARCINOMA, Gleason score 6/10 (3+3); -- Approximately 2% of tissue involved; -- Please see tumour summary. Comment: The World Health Organization (WHO) grade is: 1 out of 5.
Prostate Tissue, Transurethral Resection of Prostate (TURP): - ADENOCARCINOMA, Gleason score 7/10 (3+4); -- Approximately 4% of tissue involved; -- Please see tumour summary. - Benign inflamed urothelium. Comment: The World Health Organization (WHO) grade is: 2 out of 5. Gleason pattern 3 represents 90% of the tumour, and Gleason pattern 4 represents 10% of the tumour.
Block letters
PROSTATE TISSUE, TRANSURETHRAL RESECTION OF PROSTATE (TURP): - ADENOCARCINOMA, GLEASON SCORE 7/10 (3+4); - APPROXIMATELY 5% OF TISSUE INVOLVED; - PLEASE SEE TUMOUR SUMMARY. TUMOUR SUMMARY - TRANSURETHRAL RESECTION OF PROSTATE (TURP). PROCEDURE: TRANSURETHRAL PROSTATIC RESECTION. SPECIMEN SIZE: WEIGHT: 10 GRAMS. HISTOLOGIC TYPE: ADENOCARCINOMA (ACINAR, NOT OTHERWISE SPECIFIED). HISTOLOGIC GRADING: PRIMARY PATTERN: 3. SECONDARY PATTERN: 4 (40% OF TUMOUR). TOTAL GLEASON SCORE: 7 (3+4). TUMOUR QUANTITATION - PERCENTAGE OF PROSTATIC TISSUE INVOLVED BY TUMOUR: 80 %. PERIPROSTATIC FAT INVASION: NOT IDENTIFIED. SEMINAL VESICLE INVASION: NOT IDENTIFIED. LYMPH-VASCULAR INVASION: NOT IDENTIFIED. PERINEURAL INVASION: NOT IDENTIFIED. ADDITIONAL PATHOLOGIC FINDINGS: HIGH-GRADE PROSTATIC INTRAEPITHELIAL NEOPLASIA (HGPIN). NODULAR PROSTATIC HYPERPLASIA. CHRONIC INFLAMMATION.
PROSTATE TISSUE, TRANSURETHRAL RESECTION OF THE PROSTATE (TURP): - ADENOCARCINOMA, GLEASON SCORE 6/10 (3+3); - APPROXIMATELY 5% OF TISSUE INVOLVED; - PLEASE SEE TUMOUR SUMMARY.
Prostate Tissue, Transurethral Resection of Prostate (TURP): - Adenocarcinoma, Gleason score 7 (3+4) - Approximately 3% of sampled tissue involved - Please see tumour summary Tumour summary: Procedure: Transurethral resection of prostate Specimen weight: 11.7 g Histologic type: Adenocarcinoma, acinar type Histologic grade: Primary pattern: 3 Secondary pattern: 4 (<15% of tumor) Total Gleason score: 7/10 (3+4) Tumor volume: 3% of tissue Periprostate fat invasion: Periprostatic fat not identified Seminal vesicle invasion: Seminal vesicle not identified Lymphovascular inasion: Not identified Perineural invasion: Not identified Additional findings: Glandular and stromal hyperplasia Mild chronic inflammation
Biopsy specimens
Important elements - a list:[15]
- Type of cancer, e.g. "prostatic adenocarcinoma, acinar type".
- Gleason score including primary and secondary pattern, e.g. "Gleason score 3+4=7".
- Number of cores and number involved, e.g. "2/3 cores involved by cancer".
- Percent area involved, i.e. how much of the core is cancer, e.g. "75% of specimen is tumour". ‡
- Percent area involved that is Gleason pattern 4 or 5, e.g. "25% of the tumour is Gleason pattern 4 or 5".
- Presence of perineural invasion.
- Presence of extension into fat (extraprostatic extension).
Notes:
- ‡ "Percent area involved" may seem like an odd thing to request 'cause it is sampling dependent, i.e. if the radiologist sticks the biopsy needle deeper into the lesion more of the core is positive, but urologists think it is important -- more important than perineural invasion.[47]
- There is disagreement on how one should measure patchy cancer (cancer when there is interspersed normal). Epstein believes one should include the interspersed benign if the cancer is patchy, as the groupings of tumour likely join out of the plane of section.[48]
- A review by Epstein on the topic of tumour volume suggests it does not have predictive value in multivariante analyses.[48]
- The biopsy tumour volume is a predictor of Gleason score upgrading on prostatectomy.[49]
Completely negative
A. PROSTATE, RIGHT LATERAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. B. PROSTATE, RIGHT MEDIAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. C. PROSTATE, RIGHT LATERAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE. D. PROSTATE, RIGHT MEDIAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE. E. PROSTATE, RIGHT LATERAL INTERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. F. PROSTATE, RIGHT MEDIAL INFERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. G. PROSTATE, LEFT LATERAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. H. PROSTATE, LEFT MEDIAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. I. PROSTATE, LEFT LATERAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE. J. PROSTATE, LEFT MEDIAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE. K. PROSTATE, LEFT LATERAL INTERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. L. PROSTATE, LEFT MEDIAL INFERIOR, BIOPSY: - BENIGN PROSTATE TISSUE.
Negative biopsy in surveillance
COMMENT: The previous results are noted. The absence of cancer in this biopsy may be due to sampling.
No glands
F. PROSTATE, RIGHT MEDIAL MIDZONE, BIOPSY: - BENIGN FIBROMUSCULAR TISSUE; - NO PROSTATIC GLANDULAR TISSUE PRESENT.
Inflammation
G. PROSTATE, LEFT LATERAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE; - FOCAL CHRONIC INFLAMMATION.
F. PROSTATE, RIGHT MEDIAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE; - CHRONIC INFLAMMATION.
F. PROSTATE, RIGHT MEDIAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE; - ACUTE AND CHRONIC INFLAMMATION.
Positive
F. PROSTATE, RIGHT MEDIAL INFERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 6/10 (3+3); - 1/1 CORE INVOLVED; APPROXIMATELY 5% OF TISSUE INVOLVED.
F. PROSTATE, RIGHT MEDIAL INFERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 6/10 (3+3); - 1/1 CORE INVOLVED; APPROXIMATELY 25% OF TISSUE INVOLVED; - PERINEURAL INVASION PRESENT.
G. PROSTATE, LEFT LATERAL SUPERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 7/10 (4+3); - 1/1 CORE INVOLVED; APPROXIMATELY 5% OF TISSUE INVOLVED; - PERINEURAL INVASION PRESENT.
H. PROSTATE, LEFT MEDIAL SUPERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 8/10 (4+4); - 1/1 CORE INVOLVED; APPROXIMATELY 15% OF TISSUE INVOLVED.
H. PROSTATE, LEFT MEDIAL SUPERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 8/10 (4+4); - 1/1 CORE INVOLVED; APPROXIMATELY 15% OF TISSUE INVOLVED; - PERINEURAL INVASION PRESENT.
Tumour summaries
- These are not completely without controversy.
- It should be noted that treatment is driven by the highest Gleason score.[citation needed]
TUMOUR SUMMARY - PROSTATE CORE BIOPSIES: - HISTOLOGIC TYPE: ADENOCARCINOMA (ACINAR, NOT OTHERWISE SPECIFIED). - TOTAL GLEASON SCORE: 7. - PRIMARY PATTERN: 4. - SECONDARY PATTERN: 3. - PERCENT OF TUMOUR WITH PATTERN HIGHER THAN GRADE 3: 75%. - NUMBER OF CORES POSITIVE: 10. - TOTAL NUMBER OF CORES: 12. - TOTAL LINEAR MILLIMETERS OF NEEDLE CORE TISSUE: 152 MM. - PERCENT OF NEEDLE CORE TISSUE THAT IS TUMOUR: 44%. - PERIPROSTATIC FAT INVASION: NOT IDENTIFIED. - SEMINAL VESICLE INVASION: SEMINAL VESICLE NOT IDENTIFIED. - LYMPHOVASCULAR INVASION: NOT IDENTIFIED. - PERINEURAL INVASION: PRESENT. - ADDITIONAL FINDINGS: HIGH-GRADE PROSTATIC INTRAEPITHELIAL NEOPLASIA, CHRONIC INFLAMMATION (FOCAL).
TUMOUR SUMMARY - PROSTATE CORE BIOPSIES: - HISTOLOGIC TYPE: ADENOCARCINOMA (ACINAR, NOT OTHERWISE SPECIFIED). - HIGHEST GLEASON SCORE: 8 (4+4). - SUMMARY GLEASON SCORE: 7 (4+3). - PERCENT OF TUMOUR WITH PATTERN 4: 55%. - PERCENT OF TUMOUR WITH PATTERN 5: 0%. - NUMBER OF CORES POSITIVE: 12. - TOTAL NUMBER OF CORES: 12. - TOTAL LINEAR MILLIMETERS OF NEEDLE CORE TISSUE: 178 MM. - PERCENT OF NEEDLE CORE TISSUE THAT IS TUMOUR: 80%. - PERINEURAL INVASION: PRESENT. - PERIPROSTATIC FAT INVASION: PRESENT. - LYMPHOVASCULAR INVASION: NOT IDENTIFIED. - SEMINAL VESICLE INVASION: NOT IDENTIFIED.
Seminal vesicle/ejaculatory duct invasion on biopsy
COMMENT: The seminal vesicles and ejaculatory ducts have the same histology; thus, it is not usually possible to confidently differentiate them in a needle biopsy. SV/ED invasion was demonstrated with CK7, CK34betaE12/AMACR, PSA and p63 immunostaining. The tumour is PSA and AMACR positive.
Intraductal spread of prostate cancer
Intraductal carcinoma of the prostate
- AKA intraductal carcinoma.
- AKA intraductal prostate carcinoma.
Unusual forms of prostate cancer
Prostatic ductal adenocarcinoma
PIN-like prostatic ductal adenocarcinoma
Foamy gland carcinoma
Atrophic prostate carcinoma
- AKA atrophic carcinoma.
Mucinous prostate carcinoma
Pseudohyperplastic prostatic adenocarcinoma
- AKA pseudohyperplastic adenocarcinoma.
Prostatic signet ring cell carcinoma
General
- Very rare - 9 cases in a series of 29,783 prostate cancer cases.[51]
- Criteria vary - percentage of SRCs required for Dx varies from 20% to 50%.[51]
Microscopic
Features:
- Signet ring cells - see basics article.
DDx:
- Acinar adenocarcinoma - Gleason pattern 4 with very small glands.
Images
- Prostatic SRCC (nih.gov).
- Prostatic SRCC (nature.com).
- Prostatic SRCC (nature.com).
- Prostatic SRCC (webpathology.com) - looks like acinar adenocarcinoma.
Stains
- Alcian blue-PAS stain +ve.
- PAS -- 50% of cases +ve.[51]
- Alcian blue -- 44% of cases +ve.[51]
Sarcomatoid carcinoma of the prostate
Small cell carcinoma of the prostate gland
Adenoid cystic/basal cell carcinoma of the prostate
- Abbreviated ACBCC.
Postradiation prostate cancer
Metastatic disease and other cancers of the prostate
Urothelial carcinoma
See also
References
- ↑ Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD (August 1993). "The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients". J. Urol. 150 (2 Pt 1): 379–85. PMID 8326560.
- ↑ Li, D.; Kumaraswamy, E.; Harlan-Williams, LM.; Jensen, RA. (2013). "The role of BRCA1 and BRCA2 in prostate cancer.". Front Biosci (Landmark Ed) 18: 1445-59. PMID 23747895.
- ↑ Castro, E.; Eeles, R. (May 2012). "The role of BRCA1 and BRCA2 in prostate cancer.". Asian J Androl 14 (3): 409-14. doi:10.1038/aja.2011.150. PMID 22522501.
- ↑ Taherian, N.; Hamel, N.; Bégin, LR.; Bismar, TA.; Goldgar, DE.; Feng, BJ.; Foulkes, WD. (Feb 2013). "Familial prostate cancer: the damage done and lessons learnt.". Nat Rev Urol 10 (2): 116-22. doi:10.1038/nrurol.2012.257. PMID 23318356.
- ↑ Ip, S.; Dahabreh, IJ.; Chung, M.; Yu, WW.; Balk, EM.; Iovin, RC.; Mathew, P.; Luongo, T. et al. (Dec 2011). "An evidence review of active surveillance in men with localized prostate cancer.". Evid Rep Technol Assess (Full Rep) (204): 1-341. PMID 23126653. http://www.ncbi.nlm.nih.gov/books/NBK83054/.
- ↑ 6.0 6.1 6.2 Palisaar, JR.; Noldus, J.; Löppenberg, B.; von Bodman, C.; Sommerer, F.; Eggert, T. (Sep 2012). "Comprehensive report on prostate cancer misclassification by 16 currently used low-risk and active surveillance criteria.". BJU Int 110 (6 Pt B): E172-81. doi:10.1111/j.1464-410X.2012.10935.x. PMID 22314081.
- ↑ Klotz, L. (Nov 2005). "Active surveillance for prostate cancer: for whom?". J Clin Oncol 23 (32): 8165-9. doi:10.1200/JCO.2005.03.3134. PMID 16278468.
- ↑ URL: http://www.active-surveillance.com/laurence-klotz-md/. Accessed on: 12 July 2013.
- ↑ Klotz, L. (May 2013). "Active surveillance: patient selection.". Curr Opin Urol 23 (3): 239-44. doi:10.1097/MOU.0b013e32835f8f6b. PMID 23548978.
- ↑ Klotz, L. (2012). "Active surveillance for low-risk prostate cancer.". F1000 Med Rep 4: 16. doi:10.3410/M4-16. PMC 3412317. PMID 22891078. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3412317/.
- ↑ Fleshner, NE.; O'Sullivan, M.; Premdass, C.; Fair, WR. (Feb 1999). "Clinical significance of small (less than 0.2 cm3) hypoechoic lesions in men with normal digital rectal examinations and prostate-specific antigen levels less than 10 ng/mL.". Urology 53 (2): 356-8. PMID 9933054.
- ↑ Nakano Junqueira, VC.; Zogbi, O.; Cologna, A.; Dos Reis, RB.; Tucci, S.; Reis, LO.; Westphalen, AC.; Muglia, VF. (Oct 2012). "Is a visible (hypoechoic) lesion at biopsy an independent predictor of prostate cancer outcome?". Ultrasound Med Biol 38 (10): 1689-94. doi:10.1016/j.ultrasmedbio.2012.06.006. PMID 22920545.
- ↑ Devonec, M.; Fendler, JP.; Monsallier, M.; Mouriquand, P.; Maquet, JH.; Mestas, JL.; Dutrieux-Berger, N.; Perrin, P. (Feb 1990). "The significance of the prostatic hypoechoic area: results in 226 ultrasonically guided prostatic biopsies.". J Urol 143 (2): 316-9. PMID 1688955.
- ↑ Cindolo, L.; Benincasa, G.; Autorino, R.; Domizio, S.; De Rosa, G.; Testa, G.; D'Armiento, M.; Altieri, V.. "Prevalence of silent prostatic adenocarcinoma in 165 patients undergone cystoprostatectomy: a retrospective study.". Oncol Rep 8 (2): 269-71. PMID 11182038.
- ↑ 15.0 15.1 15.2 Humphrey PA (January 2007). "Diagnosis of adenocarcinoma in prostate needle biopsy tissue". J. Clin. Pathol. 60 (1): 35–42. doi:10.1136/jcp.2005.036442. PMC 1860598. PMID 17213347. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860598/?tool=pubmed.
- ↑ 16.0 16.1 16.2 Epstein JI (March 2004). "Diagnosis and reporting of limited adenocarcinoma of the prostate on needle biopsy". Mod. Pathol. 17 (3): 307–15. doi:10.1038/modpathol.3800050. PMID 14739905. http://www.nature.com/modpathol/journal/v17/n3/full/3800050a.html.
- ↑ URL: http://pancreaticcancer2000.com/page1.htm. Accessed on: 3 June 2010.
- ↑ 18.0 18.1 Van der Kwast, TH.; Evans, A.; Lockwood, G.; Tkachuk, D.; Bostwick, DG.; Epstein, JI.; Humphrey, PA.; Montironi, R. et al. (Feb 2010). "Variability in diagnostic opinion among pathologists for single small atypical foci in prostate biopsies.". Am J Surg Pathol 34 (2): 169-77. doi:10.1097/PAS.0b013e3181c7997b. PMID 20061936.
- ↑ Egevad, L.; Allsbrook, WC.; Epstein, JI. (Mar 2006). "Current practice of diagnosis and reporting of prostate cancer on needle biopsy among genitourinary pathologists.". Hum Pathol 37 (3): 292-7. PMID 16613324.
- ↑ 20.0 20.1 Baisden, BL.; Kahane, H.; Epstein, JI. (Aug 1999). "Perineural invasion, mucinous fibroplasia, and glomerulations: diagnostic features of limited cancer on prostate needle biopsy.". Am J Surg Pathol 23 (8): 918-24. PMID 10435561.
- ↑ Kelemen, PR.; Buschmann, RJ.; Weisz-Carrington, P. (Feb 1990). "Nucleolar prominence as a diagnostic variable in prostatic carcinoma.". Cancer 65 (4): 1017-20. PMID 1688728.
- ↑ Weedman Molavi, Diana (2008). The Practice of Surgical Pathology: A Beginner's Guide to the Diagnostic Process (1st ed.). Springer. pp. 100-3. ISBN 978-0387744858.
- ↑ Yang C, Humphrey PA (March 2020). "False-Negative Histopathologic Diagnosis of Prostatic Adenocarcinoma". Arch. Pathol. Lab. Med. 144 (3): 326–334. doi:10.5858/arpa.2019-0456-RA. PMID 31729886.
- ↑ 24.0 24.1 Lu, J.; Wirth, GJ.; Wu, S.; Chen, J.; Dahl, DM.; Olumi, AF.; Young, RH.; McDougal, WS. et al. (Jul 2012). "A close surgical margin after radical prostatectomy is an independent predictor of recurrence.". J Urol 188 (1): 91-7. doi:10.1016/j.juro.2012.02.2565. PMID 22578729.
- ↑ URL: http://urology.jhu.edu/newsletter/prostate_cancer410.php. Accessed on: 26 March 2013.
- ↑ 26.0 26.1 Steinsvik, EA.; Axcrona, K.; Angelsen, A.; Beisland, C.; Dahl, A.; Eri, LM.; Haug, ES.; Svindland, A. et al. (Aug 2012). "Does a surgeon's annual radical prostatectomy volume predict the risk of positive surgical margins and urinary incontinence at one-year follow-up? - Findings from a prospective national study.". Scand J Urol Nephrol. doi:10.3109/00365599.2012.707684. PMID 22860630.
- ↑ Koutlidis, N.; Mourey, E.; Champigneulle, J.; Mangin, P.; Cormier, L. (Jul 2012). "Robot-assisted or pure laparoscopic nerve-sparing radical prostatectomy: What is the optimal procedure for the surgical margins? A single center experience.". Int J Urol. doi:10.1111/j.1442-2042.2012.03102.x. PMID 22860572.
- ↑ Mauermann, J.; Fradet, V.; Lacombe, L.; Dujardin, T.; Tiguert, R.; Tetu, B.; Fradet, Y. (Aug 2012). "The Impact of Solitary and Multiple Positive Surgical Margins on Hard Clinical End Points in 1712 Adjuvant Treatment-Naive pT2-4 N0 Radical Prostatectomy Patients.". Eur Urol. doi:10.1016/j.eururo.2012.08.002. PMID 22901983.
- ↑ Chalfin, HJ.; Dinizo, M.; Trock, BJ.; Feng, Z.; Partin, AW.; Walsh, PC.; Humphreys, E.; Han, M. (Jul 2012). "Impact of surgical margin status on prostate-cancer-specific mortality.". BJU Int. doi:10.1111/j.1464-410X.2012.11371.x. PMID 22788795.
- ↑ 30.0 30.1 Pierorazio, PM.; Epstein, JI.; Humphreys, E.; Han, M.; Walsh, PC.; Partin, AW. (Jan 2010). "The significance of a positive bladder neck margin after radical prostatectomy: the American Joint Committee on Cancer Pathological Stage T4 designation is not warranted.". J Urol 183 (1): 151-7. doi:10.1016/j.juro.2009.08.138. PMID 19914651.
- ↑ Chung, MS.; Lee, SH.; Lee, DH.; Chung, BH. (Feb 2013). "Evaluation of the 7th American Joint Committee on cancer TNM staging system for prostate cancer in point of classification of bladder neck invasion.". Jpn J Clin Oncol 43 (2): 184-8. doi:10.1093/jjco/hys196. PMID 23225909.
- ↑ 32.0 32.1 32.2 Ali, TZ.; Epstein, JI. (Sep 2005). "Perineural involvement by benign prostatic glands on needle biopsy.". Am J Surg Pathol 29 (9): 1159-63. PMID 16096404.
- ↑ 33.0 33.1 33.2 Amin MB, Epstein JI, Ulbright TM, et al. (August 2014). "Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the international society of urological pathology consensus conference". Am. J. Surg. Pathol. 38 (8): 1017–22. doi:10.1097/PAS.0000000000000254. PMID 25025364.
- ↑ Azumi, N.; Traweek, ST.; Battifora, H. (Aug 1991). "Prostatic acid phosphatase in carcinoid tumors. Immunohistochemical and immunoblot studies.". Am J Surg Pathol 15 (8): 785-90. PMID 1712549.
- ↑ Goldstein, NS. (Mar 2002). "Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores.". Am J Clin Pathol 117 (3): 471-7. doi:10.1309/G6PR-Y774-X738-FG2K. PMID 11888088.
- ↑ URL: http://biocare.net/wp-content/uploads/225DS.pdf. Accessed on: 18 October 2011.
- ↑ URL: http://www.antibodies-online.com/antibody/308235/anti-PIN-4+p63+Cytokeratin+HMW+p504S++AMACR/. Accessed on: 18 October 2011.
- ↑ URL: http://www.webpathology.com/image.asp?case=96&n=5. Accessed on: 18 October 2011.
- ↑ Fleischmann, A.; Rocha, C.; Schobinger, S.; Seiler, R.; Wiese, B.; Thalmann, GN. (Apr 2011). "Androgen receptors are differentially expressed in Gleason patterns of prostate cancer and down-regulated in matched lymph node metastases.". Prostate 71 (5): 453-60. doi:10.1002/pros.21259. PMID 20878946.
- ↑ Boran, C.; Kandirali, E.; Yilmaz, F.; Serin, E.; Akyol, M.. "Reliability of the 34βE12, keratin 5/6, p63, bcl-2, and AMACR in the diagnosis of prostate carcinoma.". Urol Oncol 29 (6): 614-23. doi:10.1016/j.urolonc.2009.11.013. PMID 20189848.
- ↑ Langner, C.; Rupar, G.; Leibl, S.; Hutterer, G.; Chromecki, T.; Hoefler, G.; Rehak, P.; Zigeuner, R. (Mar 2006). "Alpha-methylacyl-CoA racemase (AMACR/P504S) protein expression in urothelial carcinoma of the upper urinary tract correlates with tumour progression.". Virchows Arch 448 (3): 325-30. doi:10.1007/s00428-005-0129-6. PMID 16315020.
- ↑ Googe, PB.; McGinley, KM.; Fitzgibbon, JF. (Feb 1997). "Anticytokeratin antibody 34 beta E12 staining in prostate carcinoma.". Am J Clin Pathol 107 (2): 219-23. PMID 9024071.
- ↑ Watson, K.; Wang, C.; Yilmaz, A.; Bismar, TA.; Trpkov, K. (Apr 2013). "Use of immunohistochemistry in routine workup of prostate needle biopsies: a tertiary academic institution experience.". Arch Pathol Lab Med 137 (4): 541-5. doi:10.5858/arpa.2012-0145-OA. PMID 23273390.
- ↑ Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, Gong Y, Cheng H, Laxman B, Vellaichamy A, Shankar S, Li Y, Dhanasekaran SM, Morey R, Barrette T, Lonigro RJ, Tomlins SA, Varambally S, Qin ZS, Chinnaiyan AM (May 2010). "An Integrated Network of Androgen Receptor, Polycomb, and TMPRSS2-ERG Gene Fusions in Prostate Cancer Progression". Cancer Cell 17 (5): 443–54. doi:10.1016/j.ccr.2010.03.018. PMC 2874722. PMID 20478527. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2874722/.
- ↑ 45.0 45.1 Online 'Mendelian Inheritance in Man' (OMIM) 602060
- ↑ Attard, G.; Clark, J.; Ambroisine, L.; Fisher, G.; Kovacs, G.; Flohr, P.; Berney, D.; Foster, CS. et al. (Jan 2008). "Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer.". Oncogene 27 (3): 253-63. doi:10.1038/sj.onc.1210640. PMID 17637754.
- ↑ Rubin MA, Bismar TA, Curtis S, Montie JE (July 2004). "Prostate needle biopsy reporting: how are the surgical members of the Society of Urologic Oncology using pathology reports to guide treatment of prostate cancer patients?". Am. J. Surg. Pathol. 28 (7): 946–52. PMID 15223967.
- ↑ 48.0 48.1 Epstein, JI. (Sep 2011). "Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens.". J Urol 186 (3): 790-7. doi:10.1016/j.juro.2011.02.2695. PMID 21788055.
- ↑ Fu, Q.; Moul, JW.; Bañez, LL.; Sun, L.; Mouraviev, V.; Xie, D.; Polascik, TJ. (Dec 2012). "Association between percentage of tumor involvement and Gleason score upgrading in low-risk prostate cancer.". Med Oncol 29 (5): 3339-44. doi:10.1007/s12032-012-0270-4. PMID 22688447.
- ↑ Zhao, J.; Epstein, JI. (Apr 2009). "High-grade foamy gland prostatic adenocarcinoma on biopsy or transurethral resection: a morphologic study of 55 cases.". Am J Surg Pathol 33 (4): 583-90. doi:10.1097/PAS.0b013e31818a5c6c. PMID 19033862.
- ↑ 51.0 51.1 51.2 51.3 Warner, JN.; Nakamura, LY.; Pacelli, A.; Humphreys, MR.; Castle, EP. (Dec 2010). "Primary signet ring cell carcinoma of the prostate.". Mayo Clin Proc 85 (12): 1130-6. doi:10.4065/mcp.2010.0463. PMID 21123640.