Difference between revisions of "Immunohistochemical staining"
Jensflorian (talk | contribs) (→Signal assessment: Update) |
|||
| (12 intermediate revisions by 2 users not shown) | |||
| Line 8: | Line 8: | ||
#Prognostic markers, e.g. ERBB2 (HER2). | #Prognostic markers, e.g. ERBB2 (HER2). | ||
#Proving clonality - in the context of hematologic malignancies. | #Proving clonality - in the context of hematologic malignancies. | ||
#Mutation specific antibodies, eg. [[IDH-1]] R132H. | |||
Method was introduced in 1941 by Coons.<ref>{{Cite journal | last1 = Coons | first1 = AH. | title = The development of immunohistochemistry. | journal = Ann N Y Acad Sci | volume = 177 | issue = | pages = 5-9 | month = Jun | year = 1971 | doi = | PMID = 4400556 }}</ref> | |||
==Theory== | ==Theory== | ||
| Line 18: | Line 21: | ||
**Older.<ref>{{Cite journal | last1 = Vosse | first1 = BA. | last2 = Seelentag | first2 = W. | last3 = Bachmann | first3 = A. | last4 = Bosman | first4 = FT. | last5 = Yan | first5 = P. | title = Background staining of visualization systems in immunohistochemistry: comparison of the Avidin-Biotin Complex system and the EnVision+ system. | journal = Appl Immunohistochem Mol Morphol | volume = 15 | issue = 1 | pages = 103-7 | month = Mar | year = 2007 | doi = | PMID = 17536316 }}</ref> | **Older.<ref>{{Cite journal | last1 = Vosse | first1 = BA. | last2 = Seelentag | first2 = W. | last3 = Bachmann | first3 = A. | last4 = Bosman | first4 = FT. | last5 = Yan | first5 = P. | title = Background staining of visualization systems in immunohistochemistry: comparison of the Avidin-Biotin Complex system and the EnVision+ system. | journal = Appl Immunohistochem Mol Morphol | volume = 15 | issue = 1 | pages = 103-7 | month = Mar | year = 2007 | doi = | PMID = 17536316 }}</ref> | ||
**May suffer from endogenous avidin-biotin activity.<ref name=pmid17536316>{{Cite journal | last1 = Vosse | first1 = BA. | last2 = Seelentag | first2 = W. | last3 = Bachmann | first3 = A. | last4 = Bosman | first4 = FT. | last5 = Yan | first5 = P. | title = Background staining of visualization systems in immunohistochemistry: comparison of the Avidin-Biotin Complex system and the EnVision+ system. | journal = Appl Immunohistochem Mol Morphol | volume = 15 | issue = 1 | pages = 103-7 | month = Mar | year = 2007 | doi = | PMID = 17536316 }}</ref> | **May suffer from endogenous avidin-biotin activity.<ref name=pmid17536316>{{Cite journal | last1 = Vosse | first1 = BA. | last2 = Seelentag | first2 = W. | last3 = Bachmann | first3 = A. | last4 = Bosman | first4 = FT. | last5 = Yan | first5 = P. | title = Background staining of visualization systems in immunohistochemistry: comparison of the Avidin-Biotin Complex system and the EnVision+ system. | journal = Appl Immunohistochem Mol Morphol | volume = 15 | issue = 1 | pages = 103-7 | month = Mar | year = 2007 | doi = | PMID = 17536316 }}</ref> | ||
***Higher false positive rates than with polymer based methods. | |||
*Polymer based methods. | *Polymer based methods. | ||
**Newer. | **Newer. | ||
**Less prone to false positives. | |||
***Negative controls not needed or infrequently required.<ref name=pmid24714041>{{Cite journal | last1 = Torlakovic | first1 = EE. | last2 = Francis | first2 = G. | last3 = Garratt | first3 = J. | last4 = Gilks | first4 = B. | last5 = Hyjek | first5 = E. | last6 = Ibrahim | first6 = M. | last7 = Miller | first7 = R. | last8 = Nielsen | first8 = S. | last9 = Petcu | first9 = EB. | title = Standardization of negative controls in diagnostic immunohistochemistry: recommendations from the international ad hoc expert panel. | journal = Appl Immunohistochem Mol Morphol | volume = 22 | issue = 4 | pages = 241-52 | month = Apr | year = 2014 | doi = 10.1097/PAI.0000000000000069 | PMID = 24714041 }}</ref> | |||
===Signal assessment=== | |||
*Manual (Morphology) vs. automated (Speed) counting. | |||
*Choice of proper chromogen. | |||
**Dynamic range of DAB is 1-2logs vs. fluorescent probes 2-3logs. | |||
**Protein expression range can be up to 4 logs in gene amplification (information is missed in IHC). | |||
**FastRed stains are more suited in melanocytic tumors than DAB. | |||
*Use of established cutoffs for "intensity" and for "positive" staining. | |||
*Visual pattern recogniton vs. detecting spatial subtle changes. | |||
*Detecting differences at low intensity with human eye is less accurate. | |||
==Quality control== | ==Quality control== | ||
This is an evolving area in pathology that has been ignored for a surprisingly long time. | This is an evolving area in pathology that has been ignored for a surprisingly long time. | ||
It is touched upon the in the ''[[quality]]'' article in the ''[[Quality#Immunohistochemistry|immunohistochemistry]]'' section. | It is touched upon the in the ''[[quality]]'' article in the ''[[Quality#Immunohistochemistry|immunohistochemistry]]'' section. | ||
There are at least 62 pre-analytical variables to be considered, that may affect staining results.<ref>{{Cite journal | last1 = Engel | first1 = KB. | last2 = Moore | first2 = HM. | title = Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. | journal = Arch Pathol Lab Med | volume = 135 | issue = 5 | pages = 537-43 | month = May | year = 2011 | doi = 10.1043/2010-0702-RAIR.1 | PMID = 21526952 }}</ref> | |||
==Interpretation== | ==Interpretation== | ||
| Line 46: | Line 64: | ||
In cases where the morphology is unclear, it is judicious to have two or more immunostains that support the diagnosis, and negative stains for important entities in the differential diagnosis. | In cases where the morphology is unclear, it is judicious to have two or more immunostains that support the diagnosis, and negative stains for important entities in the differential diagnosis. | ||
Publications with contradicting | Publications with contradicting results are not uncommon. Differences can arise from the fixation, processing protocol, antibody clone and interpretation. | ||
According to Galloway, one third pathologists substantially overestimate the diagnostic significance of unexpected immunohistochemical staining results.<ref name=pmid21660231>{{Cite journal | last1 = Galloway | first1 = M. | title = Base-rate error in the interpretation of immunohistochemistry. | journal = Patholog Res Int | volume = 2011 | issue = | pages = 636495 | month = | year = 2011 | doi = 10.4061/2011/636495 | PMID = 21660231 }}</ref> | According to Galloway, one third pathologists substantially overestimate the diagnostic significance of unexpected immunohistochemical staining results.<ref name=pmid21660231>{{Cite journal | last1 = Galloway | first1 = M. | title = Base-rate error in the interpretation of immunohistochemistry. | journal = Patholog Res Int | volume = 2011 | issue = | pages = 636495 | month = | year = 2011 | doi = 10.4061/2011/636495 | PMID = 21660231 }}</ref> | ||
| Line 103: | Line 121: | ||
===Breast markers=== | ===Breast markers=== | ||
*[[GCDFP-15]] ([[AKA]] BRST-2) -- specific, but NOT sensitive. | *[[GCDFP-15]] ([[AKA]] BRST-2) -- specific, but NOT sensitive. | ||
*ER (estrogen receptor) - in normal [[breast]]. | *[[Estrogen receptor|ER]] (estrogen receptor) - in normal [[breast]]. | ||
*PR (progesterone receptor) - in normal breast. | *PR (progesterone receptor) - in normal breast. | ||
*HER2/neu - pathological, assoc. with worse prognosis. | *HER2/neu - pathological, assoc. with worse prognosis. | ||
| Line 151: | Line 169: | ||
*Glypican-3. | *Glypican-3. | ||
**[[Hepatocellular carcinoma]] (HCC) stains with glypican 3, while [[liver]] with dysplastic changes and/or [[cirrhosis|cirrhotic changes]] does not.<ref>{{cite journal | author=Anatelli F, Chuang ST, Yang XJ, Wang HL. |title=Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy |journal=Am J Clin Pathol. |volume=130 |issue= 2 |pages= 219-23? |year= 2008 |pmid= 18628090 |doi= 10.1309/WMB5PX57Y4P8QCTY }}</ref> | **[[Hepatocellular carcinoma]] (HCC) stains with glypican 3, while [[liver]] with dysplastic changes and/or [[cirrhosis|cirrhotic changes]] does not.<ref>{{cite journal | author=Anatelli F, Chuang ST, Yang XJ, Wang HL. |title=Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy |journal=Am J Clin Pathol. |volume=130 |issue= 2 |pages= 219-23? |year= 2008 |pmid= 18628090 |doi= 10.1309/WMB5PX57Y4P8QCTY }}</ref> | ||
*HepPar-1 (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.<ref name=pmid12502967>The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.</ref> | *[[HepPar-1]] (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.<ref name=pmid12502967>The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.</ref> | ||
[[HCC]] vs. [[cholangiocarcinoma]]: | [[HCC]] vs. [[cholangiocarcinoma]]: | ||
| Line 312: | Line 330: | ||
*S-100 - neural differentiation, melanoma. | *S-100 - neural differentiation, melanoma. | ||
*Desmin - smooth muscle. | *Desmin - smooth muscle. | ||
*MIB1 - proliferation marker (target is Ki-67 protein). | *[[MIB1]] - proliferation marker (target is [[Ki-67]] protein). | ||
*CD99 - blue small cell tumours, membranous staining [[EWS]]. | *CD99 - blue small cell tumours, membranous staining [[EWS]]. | ||
*BCL2 - [[synovial sarcoma]], [[small cell lymphomas]], spindle cell lipoma.<ref name=Ref_DCHH107>{{Ref DCHH|107}}</ref><ref>URL: [http://ajp.amjpathol.org/cgi/content/full/160/3/759 http://ajp.amjpathol.org/cgi/content/full/160/3/759]. Accessed on: 3 August 2010.</ref> | *BCL2 - [[synovial sarcoma]], [[small cell lymphomas]], spindle cell lipoma.<ref name=Ref_DCHH107>{{Ref DCHH|107}}</ref><ref>URL: [http://ajp.amjpathol.org/cgi/content/full/160/3/759 http://ajp.amjpathol.org/cgi/content/full/160/3/759]. Accessed on: 3 August 2010.</ref> | ||
Latest revision as of 11:49, 30 November 2020
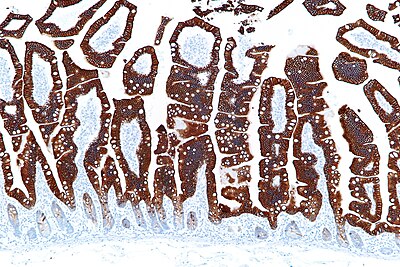
Immunohistochemical staining, also immunostaining, is a powerful tool. It is abbreviated IHC.
Utility
Use of immunohistochemistry:[1]
- Unknown primary tumours.
- Poorly differentiated tumours.
- Prognostic markers, e.g. ERBB2 (HER2).
- Proving clonality - in the context of hematologic malignancies.
- Mutation specific antibodies, eg. IDH-1 R132H.
Method was introduced in 1941 by Coons.[2]
Theory
- Antigen retrieval.
- Antibody binds to the antigen.
- Amplification - needed as the signal is usually too weak.
Signaling systems
- Avidin-biotin complex.
- Polymer based methods.
- Newer.
- Less prone to false positives.
- Negative controls not needed or infrequently required.[5]
Signal assessment
- Manual (Morphology) vs. automated (Speed) counting.
- Choice of proper chromogen.
- Dynamic range of DAB is 1-2logs vs. fluorescent probes 2-3logs.
- Protein expression range can be up to 4 logs in gene amplification (information is missed in IHC).
- FastRed stains are more suited in melanocytic tumors than DAB.
- Use of established cutoffs for "intensity" and for "positive" staining.
- Visual pattern recogniton vs. detecting spatial subtle changes.
- Detecting differences at low intensity with human eye is less accurate.
Quality control
This is an evolving area in pathology that has been ignored for a surprisingly long time.
It is touched upon the in the quality article in the immunohistochemistry section.
There are at least 62 pre-analytical variables to be considered, that may affect staining results.[6]
Interpretation
To determine whether a stain is (1) done correctly, and (2) positive, one needs to know:
- What tissues it stains:
- Tumour.
- Normal tissue.
- How it stains the various tissues:
- Patchy.
- Diffuse.
- Where it stains the various tissues:
- Nucleus.
- Cytoplasm.
- Membrane.
- A combination of the above.
Generally, interpretations can be subjective, and this is especially true when the staining is weak and focal. In other words, "... your weak [positive] stain might be somebody else’s negative."[7]
The cynical might say it is an unwritten rule that: "... if the stain is weak and focal it can be anything you want to make it -- positive or negative -- so it fits perfectly with your diagnosis!"
In cases where the morphology is unclear, it is judicious to have two or more immunostains that support the diagnosis, and negative stains for important entities in the differential diagnosis.
Publications with contradicting results are not uncommon. Differences can arise from the fixation, processing protocol, antibody clone and interpretation.
According to Galloway, one third pathologists substantially overestimate the diagnostic significance of unexpected immunohistochemical staining results.[8]
General (malignant) differential diagnosis
| Malignancy | |||||||||||||||||||||||||||||||||||||||||||||||
| Epithelial (Carcinoma) | Mesenchymal (Sarcoma) | Germ cell tumour | Neuroendocrine carcinoma | Hematologic | Malignant melanoma | ||||||||||||||||||||||||||||||||||||||||||
- Carcinoma.
- Sarcoma.
- Vimentin.
- Many pathologists think this stain is totally useless.
- Vimentin.
- Germ cell tumours.
- OCT4.
- PLAP (placental alkaline phosphatase) - not very sensitive.[9]
- Glypican 3 +ve.
- OCT4.
- Neuroendocrine carcinoma.
- Melanoma.
- Hematologic.
- Lymphoma/leukemia.
- CD45 (common leukocyte antigen).
- CD30.
- Plasma cell:
- Kappa.
- Lambda.
- CD138.
- Lymphoma/leukemia.
Keratins
Mark epithelial cells. Are typically seen in carcinomas.
Organ specific
Thyroid and lung
- TTF-1 (thyroid transcription factor-1) -- +ve in thyroid gland malignancies.
- Thyroglobulin usu. +ve in the thyroid.[15]
- Negative in classic medullary thyroid carcinoma.[16]
Image: Adenocarcinoma with nuclear TTF-1 positivity (WC).
Breast markers
- GCDFP-15 (AKA BRST-2) -- specific, but NOT sensitive.
- ER (estrogen receptor) - in normal breast.
- PR (progesterone receptor) - in normal breast.
- HER2/neu - pathological, assoc. with worse prognosis.
- HER2/neu+ cancers Tx'ed with trastuzumab (Herceptin).
Prostate gland
- PSA - prostatic-specific antigen.
- PSAP - prostatic-specific acid phosphatase.
- May be positive in hindgut neuroendocrine tumours.[17]
- p63 - stains nuclei of basal cell in normal prostate.
- 34betaE12 - stains basal cells in normal prostate.
- AMACR (racemase, P504S[18]) - present in adenocarcinoma (NOT in normal prostate).
- AR - usually present in prostate confined cancers.[19]
- CAP cocktail - AKA CAP, AKA PIN-4, AKA PIN.
- Consists of: AMACR, p63 and HMWK.
- Image: CAP cocktail (webpathology.com).
Colorectal carcinoma markers
- CK20.
- CDX2.
- Uncommon in primary lung, breast, pancreas, kidney, gallbladder, liver, urinary bladder, thyroid gland.[20]
- CEA.
Small bowel
Kidney
Xanthogranulomatous pyelonephritis:
- CD68 (for macrophages).
Ovary
Serous markers
Liver
- AFP (alpha-fetaprotein).
- Glypican-3.
- Hepatocellular carcinoma (HCC) stains with glypican 3, while liver with dysplastic changes and/or cirrhotic changes does not.[21]
- HepPar-1 (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.[22]
HCC vs. cholangiocarcinoma:
- TTF-1: ~90-100% +ve (cytoplasmic) in HCC vs. ~10% in cholangiocarcinoma.[23]
Mesothelium
Panel:[24]
- Mesothelial markers:
- Carcinoma markers:
Note:
- One should use two mesothelial markers and two carcinoma markers.[24]
Pancreas
- CK17 - approx. 50% of pancreaticobiliary adenocarcinomas & patchy.[25]
- CK19.[26]
Neuropathology
General:
- S-100.
Glial:
- GFAP.
Neuronal:
- Synaptophysin.
- Chromogranin.
Glial/Neuronal:
Specific entities:
- EMA +ve: meningioma, ependymoma (cytoplasm dot-like).[27]
- ATRX -ve: astrocytoma.
- INI1 -ve: AT/RT.
Tumour (low-grade gliomas):
- IDH-1 +ve.
- Usually negative in glioblastoma.
Miscellaneous
Macrophages
- CD68.
Special:
- S100 -- +ve in Rosai-Dorfman disease.
- CD1a -- +ve in Langerhans cell histiocytosis, Langerhans histocytes.
Special, less common:
One organ versus another
Cervix versus uterus
- Cervix (typically): CEA +ve,[33] p16 +ve.
- ... and ER -ve, PR -ve, vimentin -ve.
- Uterus (typically): vimentin +ve, ER +ve, PR +ve.[34]
- ... and CEA -ve, p16 -ve.
Liver versus bile duct
Intrahepatic cholangiocarcinoma (ICC) vs. hepatocellular carcinoma (HCC):[35]
Prostate versus bladder
Prostate adenocarcinoma vs. urothelial carcinoma:
- Prostate adenocarcinoma: PSA +ve, PSAP +ve, AR +ve, CK7 -ve, CK20 -ve, GATA3 -ve.
- Urothelial carcinoma: GATA3 +ve, CK7 +ve, CK20 +ve, PSA -ve, PSAP -ve, AR -ve.
Breast versus ovary
Breast carcinoma versus ovarian carcinoma:
Lymphomas
This is covered more extensively in the lymphoma article.
Lymphocytes
- CD45 (AKA common leukocyte antigen).
B-cells
- CD20.
- CD19 (flow only).
- PAX5.
- CD79a.
T-cells
- CD3 - general T-cell marker (marks both CD4 +ve and CD8 +ve cells).
- CD4.
- CD8.
- CD7.
- CD43.
Specific entities
Follicular lymphoma
- CD10 +ve, BCL6 +ve.
CLL
- CD5 +ve, CD23 +ve.
Mantle cell lymphoma
Hodgkin's lymphoma
This is covered more extensively in the Hodgkin lymphoma article.
Classic types:
- CD30 Reed-Sternberg cells (RSCs) +ve ~98%.[39]
- CD15 Reed-Sternberg cells +ve ~80%, stains neutrophils.
Germ cell tumours
Seminoma
- D2-40 +ve.[9]
- OCT4 +ve.
Embryonal carcinoma
- CD30 +ve - cytoplasm, cell membrane, Golgi.
- Rarely positive in seminoma.
- CK7 +ve.[40]
- AE1/AE3 +ve.
Yolk sac tumour (endodermal sinus tumour)
- AFP (alpha fetoprotein).
Choriocarcinoma
- beta-hCG.
Bare bones mnemonic for GCTs
The germ cell tumour (GCT) IHC mnemonic ABCD:
- AFP = yolk sac tumour.
- Beta-hCG = choriocarcinoma.
- CD30 = embryonal carcinoma.
- D2-40 = seminoma.
Spindle cell lesions
Abbreviated spindle cell panel (memory device SCADS):
- S100.
- CD34.
- AE1/AE3.
- Desmin.
- SMA.
A MFH panel:
- CD34 - GIST, angiosarcoma, Kaposi sarcoma, solitary fibrous tumour/hemangiopericytoma, dermatofibrosarcoma protuberans (DFSP), spindle cell lipoma.
- S-100 - neural differentiation, melanoma.
- Desmin - smooth muscle.
- MIB1 - proliferation marker (target is Ki-67 protein).
- CD99 - blue small cell tumours, membranous staining EWS.
- BCL2 - synovial sarcoma, small cell lymphomas, spindle cell lipoma.[41][42]
- PGP 9.5.
- SMMS - smooth muscle.
- Caldesmon - muscle.
- PDGFR - GIST.
- STAT6 - Hemangiopericytoma/SFT.
Muscle markers
- Desmin - all three types.
- H-caldesmon - smooth muscle - most specific.
- Smooth muscle actin (SMA) - smooth muscle.
- MyoD1 - skeletal muscle.
- Smooth muscle myosin (abbreviated SMMS).
Proliferation markers
- MIB1 - an antibody against the protein Ki-67 (a protein expressed in proliferating cells).
Notes:
- Ki-67 is found in lymphocytes - these are a positive control; lymphocytes should not be confused for (positive) tumour cells.[43]
- MIB1 should not be confused with mindbomb homolog 1 (MIB-1), a gene that regulates apoptosis.[44]
Carcinomas
CK7 and CK20
CK7+ CK20-
- Ovary (but not mucinous).
- Breast.
- Endometrial.
- Lung (adenocarcinoma).
- Mesothelioma.
- Salivary gland.
- Thyroid gland (all).
Mnemonic: OBE + lung x2 + H&N x2
CK7- CK20+
CK7+ CK20+
- Pancreatic adenocarcinoma.
- Ovary, mucinous subtype.
- Occasionally gastric adenocarcinoma, cholangiocarcinoma.
- "Transitional cell carcinoma" (urothelial cell carcinoma).§
- Esophageal adenocarcinoma.
Mnemonic: POOTE.
Note:
- § - Transitional cell carcinoma of the ovary is usu. CK20 -ve.[45]
CK7- CK20-
- Neuroendocrine lung (small cell carcinoma).
- Adrenocortical carcinoma (ACC).
- Squamous cell carcinoma (all sites of the body).
- Hepatocellular carcinoma (HCC).
- Thymoma.
- Urogenital tumours - germ cell tumours.
- Renal cell carcinoma (clear cell type).
- Prostate adenocarcinoma.
Mnemonic: NASH TURP.
Vimentin and cytokeratin positivity
A few tumours are positive for both vimentin and cytokeratins.
Sarcomas and cytokeratins
Most sarcomas are cytokeratin negative.
Exceptions - classic:
- Angiosarcoma, epithelioid.
- Synovial sarcoma.
- Chordoma.
- Desmoplastic small round cell tumour.
- Epithelioid sarcoma.
Others:
See also
References
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 175. ISBN 978-1416054542.
- ↑ Coons, AH. (Jun 1971). "The development of immunohistochemistry.". Ann N Y Acad Sci 177: 5-9. PMID 4400556.
- ↑ Vosse, BA.; Seelentag, W.; Bachmann, A.; Bosman, FT.; Yan, P. (Mar 2007). "Background staining of visualization systems in immunohistochemistry: comparison of the Avidin-Biotin Complex system and the EnVision+ system.". Appl Immunohistochem Mol Morphol 15 (1): 103-7. PMID 17536316.
- ↑ Vosse, BA.; Seelentag, W.; Bachmann, A.; Bosman, FT.; Yan, P. (Mar 2007). "Background staining of visualization systems in immunohistochemistry: comparison of the Avidin-Biotin Complex system and the EnVision+ system.". Appl Immunohistochem Mol Morphol 15 (1): 103-7. PMID 17536316.
- ↑ Torlakovic, EE.; Francis, G.; Garratt, J.; Gilks, B.; Hyjek, E.; Ibrahim, M.; Miller, R.; Nielsen, S. et al. (Apr 2014). "Standardization of negative controls in diagnostic immunohistochemistry: recommendations from the international ad hoc expert panel.". Appl Immunohistochem Mol Morphol 22 (4): 241-52. doi:10.1097/PAI.0000000000000069. PMID 24714041.
- ↑ Engel, KB.; Moore, HM. (May 2011). "Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue.". Arch Pathol Lab Med 135 (5): 537-43. doi:10.1043/2010-0702-RAIR.1. PMID 21526952.
- ↑ URL: http://bitesizebio.com/articles/immunohistochemistry-getting-the-stain-you-want/. Accessed on: 1 September 2012.
- ↑ Galloway, M. (2011). "Base-rate error in the interpretation of immunohistochemistry.". Patholog Res Int 2011: 636495. doi:10.4061/2011/636495. PMID 21660231.
- ↑ 9.0 9.1 Iczkowski KA, Butler SL, Shanks JH, et al (February 2008). "Trials of new germ cell immunohistochemical stains in 93 extragonadal and metastatic germ cell tumors". Hum. Pathol. 39 (2): 275-81. doi:10.1016/j.humpath.2007.07.002. PMID 18045648.
- ↑ Kurokawa, M.; Nabeshima, K.; Akiyama, Y.; Maeda, S.; Nishida, T.; Nakayama, F.; Amano, M.; Ogata, K. et al. (May 2003). "CD56: a useful marker for diagnosing Merkel cell carcinoma.". J Dermatol Sci 31 (3): 219-24. PMID 12727026.
- ↑ Jani P, Chetty R, Ghazarian DM (April 2008). "An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature". Am J Dermatopathol 30 (2): 174–7. doi:10.1097/DAD.0b013e318165b8fe. PMID 18360125.
- ↑ URL: http://www.ncbi.nlm.nih.gov/omim/156845. Accessed on: 18 August 2010.
- ↑ Roma, AA.; Magi-Galluzzi, C.; Zhou, M. (Jan 2007). "Differential expression of melanocytic markers in myoid, lipomatous, and vascular components of renal angiomyolipomas.". Arch Pathol Lab Med 131 (1): 122-5. doi:10.1043/1543-2165(2007)131[122:DEOMMI]2.0.CO;2. PMID 17227112.
- ↑ Jagirdar J (March 2008). "Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung". Arch. Pathol. Lab. Med. 132 (3): 384–96. PMID 18318581. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=132&page=384.
- ↑ Dralle, H.; Böcker, W. (1982). "[Thyroglobulin immunohistochemistry: new aspects of pathophysiology and differential diagnosis of benign and malignant goitre (author's transl)].". Langenbecks Arch Chir 356 (3): 205-12. PMID 7070163.
- ↑ de Micco, C.; Chapel, F.; Dor, AM.; Garcia, S.; Ruf, J.; Carayon, P.; Henry, JF.; Lebreuil, G. (Mar 1993). "Thyroglobulin in medullary thyroid carcinoma: immunohistochemical study with polyclonal and monoclonal antibodies.". Hum Pathol 24 (3): 256-62. PMID 8454270.
- ↑ Azumi, N.; Traweek, ST.; Battifora, H. (Aug 1991). "Prostatic acid phosphatase in carcinoid tumors. Immunohistochemical and immunoblot studies.". Am J Surg Pathol 15 (8): 785-90. PMID 1712549.
- ↑ http://www.antibodies-online.com/antibody/125649/P504S+alphaMethylacylCoA+Racemace+AMACR+Human/
- ↑ Fleischmann, A.; Rocha, C.; Schobinger, S.; Seiler, R.; Wiese, B.; Thalmann, GN. (Apr 2011). "Androgen receptors are differentially expressed in Gleason patterns of prostate cancer and down-regulated in matched lymph node metastases.". Prostate 71 (5): 453-60. doi:10.1002/pros.21259. PMID 20878946.
- ↑ Kaimaktchiev, V.; Terracciano, L.; Tornillo, L.; Spichtin, H.; Stoios, D.; Bundi, M.; Korcheva, V.; Mirlacher, M. et al. (Nov 2004). "The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas.". Mod Pathol 17 (11): 1392-9. doi:10.1038/modpathol.3800205. PMID 15205684.
- ↑ Anatelli F, Chuang ST, Yang XJ, Wang HL. (2008). "Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy". Am J Clin Pathol. 130 (2): 219-23?. doi:10.1309/WMB5PX57Y4P8QCTY. PMID 18628090.
- ↑ The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.
- ↑ Lei JY, Bourne PA, diSant'Agnese PA, Huang J (April 2006). "Cytoplasmic staining of TTF-1 in the differential diagnosis of hepatocellular carcinoma vs cholangiocarcinoma and metastatic carcinoma of the liver". Am. J. Clin. Pathol. 125 (4): 519–25. doi:10.1309/59TN-EFAL-UL5W-J94M. PMID 16627262.
- ↑ 24.0 24.1 Marchevsky AM (March 2008). "Application of immunohistochemistry to the diagnosis of malignant mesothelioma". Arch. Pathol. Lab. Med. 132 (3): 397-401. PMID 18318582. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=132&page=397.
- ↑ Goldstein NS, Bassi D (May 2001). "Cytokeratins 7, 17, and 20 reactivity in pancreatic and ampulla of vater adenocarcinomas. Percentage of positivity and distribution is affected by the cut-point threshold". Am. J. Clin. Pathol. 115 (5): 695–702. doi:10.1309/1NCM-46QX-3B5T-7XHR. PMID 11345833.
- ↑ Geller SA, Dhall D, Alsabeh R (March 2008). "Application of immunohistochemistry to liver and gastrointestinal neoplasms: liver, stomach, colon, and pancreas". Arch. Pathol. Lab. Med. 132 (3): 490–9. PMID 18318589.
- ↑ Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 12. ISBN 978-0443069826.
- ↑ Pernick NL, DaSilva M, Gangi MD, Crissman J, Adsay V (November 1999). ""Histiocytic markers" in melanoma". Mod. Pathol. 12 (11): 1072–7. PMID 10574605.
- ↑ URL: http://www.ncbi.nlm.nih.gov/omim/605545. Accessed on: 3 February 2011.
- ↑ URL: http://www.abcam.com/Macrophage-antibody-MAC387-FITC-ab7429.html. Accessed on: 3 February 2011.
- ↑ URL: http://www.abcam.com/Macrophage-antibody-MAC387-ab49408.html. Accessed on: 3 February 2011.
- ↑ URL: http://www.ncbi.nlm.nih.gov/omim/604862. Accessed on: 2 February 2011.
- ↑ Alkushi A, Irving J, Hsu F, et al. (March 2003). "Immunoprofile of cervical and endometrial adenocarcinomas using a tissue microarray". Virchows Arch. 442 (3): 271-7. doi:10.1007/s00428-002-0752-4. PMID 12647218.
- ↑ URL: http://www.nature.com/modpathol/journal/v19/n8/full/3800620a.html
- ↑ [Evaluation of immunohistochemical markers for differential diagnosis of hepatocellular carcinoma from intrahepatic cholangiocarcinoma] Dong H, Cong WL, Zhu ZZ, Wang B, Xian ZH, Yu H. Zhonghua Zhong Liu Za Zhi. 2008 Sep;30(9):702-5. Chinese. PMID 19173916.
- ↑ Nonaka, D.; Chiriboga, L.; Soslow, RA. (Oct 2008). "Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas.". Am J Surg Pathol 32 (10): 1566-71. doi:10.1097/PAS.0b013e31816d71ad. PMID 18724243.
- ↑ Kanner, WA.; Galgano, MT.; Stoler, MH.; Mills, SE.; Atkins, KA. (Oct 2008). "Distinguishing breast carcinoma from Müllerian serous carcinoma with mammaglobin and mesothelin.". Int J Gynecol Pathol 27 (4): 491-5. doi:10.1097/PGP.0b013e31817d5340. PMID 18753974.
- ↑ URL: http://atlasgeneticsoncology.org/Genes/BCL1.html. Accessed on: 17 December 2010.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 567. ISBN 978-0781765275.
- ↑ Cheville JC, Rao S, Iczkowski KA, Lohse CM, Pankratz VS (April 2000). "Cytokeratin expression in seminoma of the human testis". Am. J. Clin. Pathol. 113 (4): 583–8. doi:10.1309/3QLC-5MF1-JYXU-A5XX. PMID 10761461.
- ↑ Tadrous, Paul.J. Diagnostic Criteria Handbook in Histopathology: A Surgical Pathology Vade Mecum (1st ed.). Wiley. pp. 107. ISBN 978-0470519035.
- ↑ URL: http://ajp.amjpathol.org/cgi/content/full/160/3/759. Accessed on: 3 August 2010.
- ↑ Hadzi-Mihailovic, M.; Cakic, S.; Jankovic, S.; Raybaud, H.; Nedeljkovic, N.; Jankovic, L.. "Ki-67 expression in oral lichen planus.". J BUON 17 (1): 132-7. PMID 22517707.
- ↑ http://www.genenames.org/data/hgnc_data.php?hgnc_id=21086
- ↑ Tazi, EM.; Lalya, I.; Tazi, MF.; Ahellal, Y.; M'rabti, H.; Errihani, H. (2010). "Transitional cell carcinoma of the ovary: a rare case and review of literature.". World J Surg Oncol 8: 98. doi:10.1186/1477-7819-8-98. PMID 21073751.