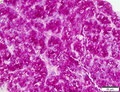
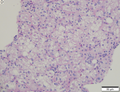
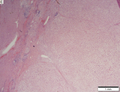
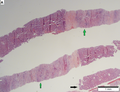
Hepatocellular carcinoma
| Hepatocellular carcinoma | |
|---|---|
| Diagnosis in short | |
 Hepatocellular carcinoma. | |
|
| |
| LM | architectural changes - liver plate thickness >3 cells thick, +/-nuclear changes of malignancy (very common), variable architecture (pseudoglandular, trabecular, fibrolamellar, solid) |
| Subtypes | sclerosing HCC, fibrolamellar HCC |
| LM DDx | cholangiocarcinoma, occasionally liver metastasis, high-grade dysplasia |
| Stains | reticulin (thickened liver plate) |
| IHC | CD34 +ve sinusoids, HepPar-1 +ve (usu.), AFP +ve (usu.), CK8/18 +ve, glypican-3 +ve |
| Gross | usu. cirrhosis (micronodular or macronodular) |
| Site | liver - see liver neoplasms |
|
| |
| Associated Dx | causes of cirrhosis, e.g. chronic alcoholism, Hepatitis C, Hepatitis B, hereditary hemochromatosis, others |
| Prevalence | most common malignant primary liver tumour, less common than metastases |
| Blood work | +/-AFP elevation |
| Prognosis | moderate to poor |
| Clin. DDx | liver metastasis, other liver tumours |
Hepatocellular carcinoma, abbreviated HCC, is the most common malignant primary liver tumour. It most often arises in the context of cirrhosis.
General
Clinical:
- Serum AFP elevated - in approx. 50% of patients.[1]
- Treatments: RFA (radiofrequency ablation), ethanol ablation, liver resection, liver transplant.[2]
- Mean survival at time of diagnosis ~6 months.[2]
Epidemiology:
- Highest where prevalence of hepatitis B virus (HBV) is high.[3]
- HCC generally arises in the setting of cirrhosis.
- Cirrhosis may be regressed and therefore not appreciated.
HCCs without cirrhosis:
- Hepatitis B virus.[3]
- Hemochromatosis.
- Fibrolamellar HCC.
- Chronic alcoholism.
- Hepatitis C virus (HCV) - chronic infection.
- Hepatitis B virus (HBV) - chronic infection.
- Aflatoxins (food contaminant - mould).[2]
- Hereditary tyrosinemia.
- Hereditary hemochromatosis.
Gross
Features:[5]
- Unifocal, multifocal or diffusely infiltrative.
- Pale in relation to surrounding liver or green (due to bile secretion).
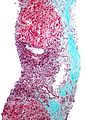
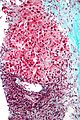
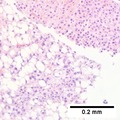
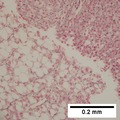
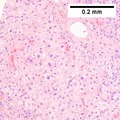
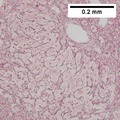
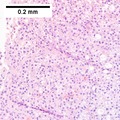
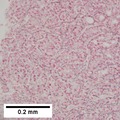
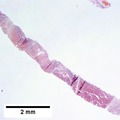
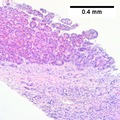
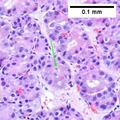
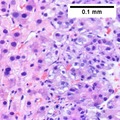
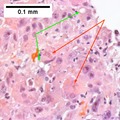
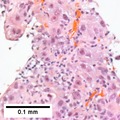
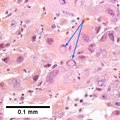
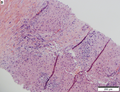
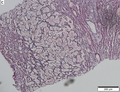
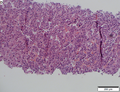
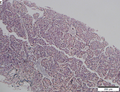
Images
Microscopic
Requirements:[8]
- Architectural changes.
- Liver plate more than 3 cells thick - key feature.
- Loss of reticulin scaffold - incomplete loss is considered significant.
- CD34+ staining cells, suggesting loss of epithelial cells that form the sinusoids.
- Loss of structures seen in a normal liver lobule (bile ductules, portal triad).
- Invasion of the portal tract - useful in well-diff. lesions.[9]
Additional findings:[10]
- Nuclear changes.
- Increased NC ratio - key feature if present.
- Nuclear hyperchromasia.
- Abnormal nuclear contour.
- Mitoses.
- Cytoplasmic changes.
- Cytoplasmic hyperchromasia, clearing or lighter staining.
Varied architecture - may be:[11]
- Pseudoglandular - can be confused with adenocarcinoma.
- Trabecular.
- Fibrolamellar.
- Solid.
Notes:
- HCC with trabecular morphology has some resemblance to normal liver - but has extra cells.
- Fibrolamellar - better prognosis, classically in young adults.
- Stroma is usually scant.[12]
ASIDE:
- Trabecula = little beam.
DDx:
- Cholangiocarcinoma.
- Combined HCC-CC.[13]
- High-grade dysplasia.
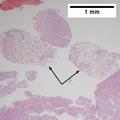
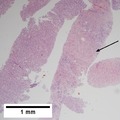
Images
Set 1
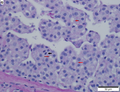
Hepatocellular carcinoma with fragments of liver afflicted by steatosis & with regenerative, benign fragments. A. Fragments of liver with crowded nuclei, consistent with hepatoma, as well as with steatosis [arrows]. B. Fragments of liver with crowded nuclei, consistent with hepatoma, as well as with larger amount of cytoplasm, consistent with regeneration [arrow]. C. The fatty liver below has aberrant-appearing nuclei, but not particularly crowded, in contrast to the fragment on top, which has crowded nuclei. D. Reticulin stain shows apparent expansion of nuclei between black lines on the fatty liver, but this may also be seen with simple steatosis; on the crowded liver note the lack of definite expansion. E. The regenerative focus shows abundant cytoplasm. F. Reticulin on this regenerative focus shows preservation of the low number of nuclei between black lines. G. This typical hepatocellular focus shows crowding, occasional acini, and basophilia, similar to the focus seen in row 2. Note that some cells show apparent steatosis. H. Reticulin on this hepatocellular focus shows loss of reticulin fibers, with more than six nuclei between several black lines (Row 4 Right 200X).
Set 2
Hepatocellular carcinoma with acini. A. Fibrous bands dissect hepatocyte nodules, mostly hepatoma. B. The fibrous band on right bears proliferating bile ductules; acinar arrangement on left shows holes much larger than canaliculi. C. The tumor has cancerous nuclei; note the bile which makes for absolute diagnostic certainty [arrow]. D. Noncancerous hepatocytes on left can be compared with tumor cells on right. Note increased nuclear crowding & a subtle increment in cytoplasmic basophilia in tumor.
Set 3
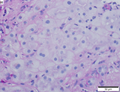
Hepatocellular carcinoma, steatohepatitic variant. A. Fragments of tumor at low power mimic normal hepatocyte groups without portal triads. B. Vacuoles, mostly small, occasionally become large enough to warrant the term “macrovesicular” [green arrows]. Note Mallory hyalin [red arrows]. D. Chronic inflammatory cells bound some cancer cells. D. Nuclear inclusions [arrows] are occasionally prominent [arrows].
Set 4
Hepatocellular carcinoma, clear cell variant. Much more difficult on biopsy than on resection is the specific identification of the clear cell variant, although it was done here. Variants are of little clinical significance apart from the fibrolamellar variant. A. Tumor fragment masses with clearing contrast with pink liver fragments. B. Tumor cell cytoplasm on the core biopsy varies, often clear, often light pink and foamy. C. PAS without diastase shows brightly positive tumor cytoplasm. D. Modestly variable, rounded nuclei show open chromatin and nucleoli. Individual pyknotic nuclei (black arrows) are insufficient as evidence of necrosis sufficient to increase grade. D. PAS with diastase shows the material is glycogen. E. At resection, the cytoplasmic clarity at low power is more impressive than on biopsy. F. The high power view of the resection specimen shows the cytoplasm is truly clear. Arrows point to Mallory bodies.
Set 5
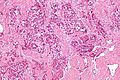
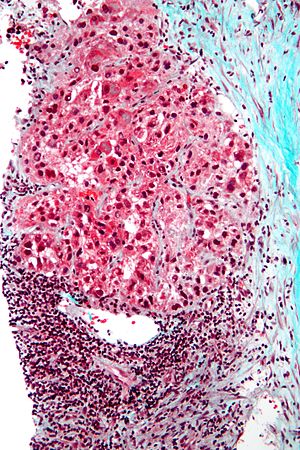
Hepatocellular carcinoma superimposed on cirrhosis in a 60 year old man. A. Dark tumor, sometimes with separate cords visible at low power (black arrow), contrasts with lightly colored regenerative nodules (green arrows) within fibrotic bands. B. Regenerative nodules are separated by a band with proliferated bile ducts, whose orientation, lacking haphazard spread, bespeaks a benign process. C. Reticulin of regenerative nodule shows two cell thick cords lacking orientation, disrupted without reason, aggregated and, at left, slighely dispersed. D. Hepatocellular carcinoma shows darker cytoplasm and more nuclei per square mm than regenerative nodules. Note the many acini, also a good sign of cancer. E. Reticulin fibers have disappeared, leaving cords with 6 or more nuclei thick in places. F. PAS D stain shows benign proliferated bile ducts, whose orientation from lower left to upper right deprives the proliferation of the haphazard nature of a cholangiocarcinoma. G. PAS-D shows the cytoplasmic digestion of glycogen in a hepatocellular carcinoma, a helpful hint at times. Note the finger like cords, the mitosis (black arrow), and the occasional acinar lumens (red arrows). H. PAS D of regenerative nodule contrasts with the image of the hepatocellular carcinoma.
Fibrolamellar hepatocellular carcinoma
- Abbreviated fibrolamellar HCC, FL-HCC, and FHCC.
General
- Rare variant.
- Classically afflicts younger patients.
- Mean age at onset ~27 years in one study.[14]
- Individuals usually do not have the classic risk factors for HCC, i.e. no cirrhosis, no hepatitis.[14]
Clinical:
- AFP usu. not elevated.[14]
Microscopic
Features:[15]
- Large polygonal tumours cells with:
- Graunular eosinophilic cytoplasm.
- Low NC ratio.[16]
- Layered dense collagen bundles.
DDx:
Note:
- If conventional HCC is seen focally within the tumour, it is conventional HCC.
Images
Sclerosing HCC
Features:
- Fibrosis. (???)
Notes:
- Seen in non-cirrhotic livers.
Grading
Edmondson-Steiner grading system:[17][18]
- Well-differentiated.
- Some say "it cannot be diagnosed on biopsy,"[19] as it cannot be reliably differentiated from a regenerative nodule.
- Moderately differentiated.
- Round, regular nuclei, some hyperchromatism, nucleoli present, increase NC ratio.
- Poor differentiated.
- Very prominent nucleoli, pronounced nuclear irregularity.
- Undifferentiated.
- Anaplastic giant cells.
Simplified description - based on MacSween:[18]
- Well-differentiated = cytologically near normal.
- Moderate = looks like a cancer, small nucleoli.
- Poor = bad cancer, raisin-like (irregular) nuclear membrane, large nucleoli (~1/3 of nucleus).
- Undifferentiated = death on a slide, huge cells (3-4x the size of other cells).
IHC
- CD34 +ve sinusoids; sinusoids in normal liver are CD34 -ve.
- HepPar-1 +ve; may be neg. in high grade tumours.
- AFP +ve; may be neg. even if the serum AFP is elevated.
- CK8/18 +ve.[20]
- Glypican-3 +ve (cytoplasmic, granular cytoplasmic or membranous).[21]
- TTF-1 +ve cytoplasmic staining.[22]
- Benign liver also has cytoplasmic staining.
Bile canaliculi:
Image:
Sign out
Without background cirrhosis
Liver Mass, Core Biopsy: - HEPATOCELLULAR CARCINOMA, moderately differentiated, see comment. Comment: The liver parenchyma has architectural changes. A reticulin stain shows a thickened liver plate. A CD34 abnormally marks the sinusoids. A trichrome stain shows minimal fibrous tissue. A HepPar-1 marks the liver tissue. A CK19 and AFP are NEGATIVE.
Negative core biopsy
LIVER CORE, BIOPSY: - CIRRHOSIS. - HEPATOCYTE CYTOLOGY WITHIN NORMAL LIMITS.
See also
References
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 588. ISBN 978-0443066573.
- ↑ 2.0 2.1 2.2 2.3 http://emedicine.medscape.com/article/282814-overview
- ↑ 3.0 3.1 3.2 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 924. ISBN 0-7216-0187-1.
- ↑ Leong TY, Leong AS (2005). "Epidemiology and carcinogenesis of hepatocellular carcinoma". HPB (Oxford) 7 (1): 5–15. doi:10.1080/13651820410024021. PMC 2023917. PMID 18333156. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2023917/.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 925. ISBN 0-7216-0187-1.
- ↑ Yusuf MA, Badar F, Meerza F, et al. (2007). "Survival from hepatocellular carcinoma at a cancer hospital in Pakistan". Asian Pac. J. Cancer Prev. 8 (2): 272–4. PMID 17696722.
- ↑ Sharieff S, Burney KA, Ahmad N, Salam A, Siddiqui T (October 2001). "Radiological features of hepatocellular carcinoma in Southern Pakistan". Trop Doct 31 (4): 224–5. PMID 11676064.
- ↑ Adapted from STC (19 Jan 2009).
- ↑ Kojiro, M.; Wanless, IR.; Alves, V.; Badve, S.; Balabaud, C.; Bedossa, P.; Bhathal, P.; Bioulac-Sage, P. et al. (Feb 2009). "Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia.". Hepatology 49 (2): 658-64. doi:10.1002/hep.22709. PMID 19177576. http://onlinelibrary.wiley.com/doi/10.1002/hep.22709/pdf.
- ↑ Adapted from STC (19 Jan 2009).
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 590-1. ISBN 978-0443066573.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 591. ISBN 978-0443066573.
- ↑ Walther, Z.; Jain, D. (2011). "Molecular pathology of hepatic neoplasms: classification and clinical significance.". Patholog Res Int 2011: 403929. doi:10.4061/2011/403929. PMC 3090128. PMID 21559202. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3090128/.
- ↑ 14.0 14.1 14.2 Stipa, F.; Yoon, SS.; Liau, KH.; Fong, Y.; Jarnagin, WR.; D'Angelica, M.; Abou-Alfa, G.; Blumgart, LH. et al. (Mar 2006). "Outcome of patients with fibrolamellar hepatocellular carcinoma.". Cancer 106 (6): 1331-8. doi:10.1002/cncr.21703. PMID 16475212.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 595-6. ISBN 978-0443066573.
- ↑ STC. 6 December 2010.
- ↑ Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. EDMONDSON HA, STEINER PE. Cancer. 1954 May;7(3):462-503. PMID 13160935.
- ↑ 18.0 18.1 Burt, Alastair D.;Portmann, Bernard C.;Ferrell, Linda D. (2006). MacSween's Pathology of the Liver (5th ed.). Churchill Livingstone. pp. 783. ISBN 978-0-443-10012-3.
- ↑ Pollet A. 28 May 2009.
- ↑ Stroescu, C.; Herlea, V.; Dragnea, A.; Popescu, I. (Mar 2006). "The diagnostic value of cytokeratins and carcinoembryonic antigen immunostaining in differentiating hepatocellular carcinomas from intrahepatic cholangiocarcinomas.". J Gastrointestin Liver Dis 15 (1): 9-14. PMID 16680226.
- ↑ Shirakawa, H.; Kuronuma, T.; Nishimura, Y.; Hasebe, T.; Nakano, M.; Gotohda, N.; Takahashi, S.; Nakagohri, T. et al. (Mar 2009). "Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer.". Int J Oncol 34 (3): 649-56. PMID 19212669. http://www.spandidos-publications.com/serveFile/ijo_34_3_649_PDF.pdf?type=article&article_id=ijo_34_3_649&item=PDF.
- ↑ Mishra, M.; Morgan, V.; Hamati, AK.; Al-Abbadi, M. (Jan 2012). "Carcinoma of unknown primary: check the liver... thanks to TTF-1.". Tenn Med 105 (1): 35-6, 40. PMID 22359993.
- ↑ Shousha, S.; Gadir, F.; Peston, D.; Bansi, D.; Thillainaygam, AV.; Murray-Lyon, IM. (Oct 2004). "CD10 immunostaining of bile canaliculi in liver biopsies: change of staining pattern with the development of cirrhosis.". Histopathology 45 (4): 335-42. doi:10.1111/j.1365-2559.2004.01927.x. PMID 15469471.
- ↑ Porcell, AI.; De Young, BR.; Proca, DM.; Frankel, WL. (Jul 2000). "Immunohistochemical analysis of hepatocellular and adenocarcinoma in the liver: MOC31 compares favorably with other putative markers.". Mod Pathol 13 (7): 773-8. PMID 10912937.
- ↑ Goodman, ZD. (Feb 2007). "Neoplasms of the liver.". Mod Pathol 20 Suppl 1: S49-60. doi:10.1038/modpathol.3800682. PMID 17486052.