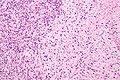
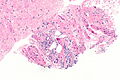
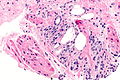
Difference between revisions of "Prostate cancer"
| Line 907: | Line 907: | ||
==Foamy gland carcinoma== | ==Foamy gland carcinoma== | ||
*[[AKA]] ''foamy gland adenocarcinoma''.<ref name=pmid19033862/> | *[[AKA]] ''foamy gland adenocarcinoma''.<ref name=pmid19033862>{{Cite journal | last1 = Zhao | first1 = J. | last2 = Epstein | first2 = JI. | title = High-grade foamy gland prostatic adenocarcinoma on biopsy or transurethral resection: a morphologic study of 55 cases. | journal = Am J Surg Pathol | volume = 33 | issue = 4 | pages = 583-90 | month = Apr | year = 2009 | doi = 10.1097/PAS.0b013e31818a5c6c | PMID = 19033862 }}</ref> | ||
{{Main|Foamy gland carcinoma}} | {{Main|Foamy gland carcinoma}} | ||
Revision as of 02:12, 23 October 2014
| Prostate carcinoma | |
|---|---|
| Diagnosis in short | |
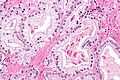
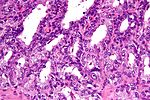
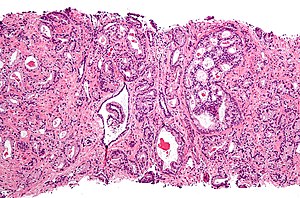 Prostate carcinoma. H&E stain. | |
|
| |
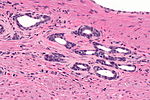
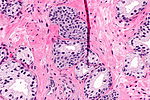
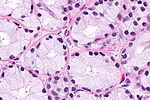
| LM | major criteria: abnormal architecture (increased gland density, usu. small circular glands, "infiltrative growth" pattern), basal cells lost, cytological abnormalities (nuclear enlargement, nucleoli); minor criteria: nuclear hyperchromasia, wispy blue mucin, pink amorphous secretions, intraluminal crystalloid, amphophilic cytoplasm, adjacent HGPIN, mitoses |
| LM DDx | high-grade prostatic intraepithelial neoplasia, atypical small acinar proliferation (biopsy only), prostatic atrophy, seminal vesicle, basal cell hyperplasia, others |
| IHC | PSA +ve, PSAP +ve, AMACR +ve, p63 -ve, CK34betaE12 -ve |
| Molecular | +/-BRCA1 mutation (genetic predisposition), +/-BRCA2 mutation (genetic predisposition) |
| Gross | usu. posterior aspect of the prostate - often not apparent at gross |
| Grossing notes | prostate biopsy, prostate chips, radical prostatectomy |
| Site | prostate gland |
|
| |
| Signs | firm, nodular prostate on digital rectal exam |
| Symptoms | often asymptomatic |
| Prevalence | very common |
| Blood work | PSA elevated (common) |
| Radiology | hypoechoic areas, no apparent abnormality |
| Prognosis | good-to-poor (depends on Gleason score and stage) |
| Clin. DDx | prostatitis, nodular hyperplasia of the prostate |
| Treatment | observation (common for low-grade, low tumour burden), radiation or radical prostatectomy |
This article deals with prostate cancer.
The vast majority of prostate cancers are carcinomas and could be labelled prostatic carcinoma. Most prostatic carcinomas are gland forming; thus, they can be labelled prostatic adenocarcinoma or adenocarcinoma of the prostate.
Benign pathology of the prostate gland, and prostate histology and anatomy are dealt with in the prostate gland article.
Conventional prostate cancer
General
- Very common.
- Increasing incidence with age - the age in years is an approximation of the percentage of men with prostate cancer.[1][citation needed]
- Usually an indolent course - most old men die with prostate cancer not from prostate cancer.
- Risk increased with a BRCA1 or BRCA2 mutation[2] - families have a mix of breast cancer and prostate cancer.
Management
Dirty first approximation
- The management changes between Gleason score 6, 7 and 8.
Typically, the implications are:
- Gleason 6: observation or radioactive seeds; surgery if patient wants.
- Gleason 7: do something -- surgery or radiation therapy.
- Gleason 8+: bad cancer -- do something quickly!
Bottom line:
- You want to be sure when you call something Gleason pattern 4.
Observational strategies
- Delay of definitive treatment (surgery or radiation).
- Common in the management of prostate cancer.
Classification:[5]
- Active surveillance (AS).
- Low risk of progression.
- May get definitive treatment later.
- Watchful waiting (WW).
- Higher risk of progression.
Note:
- There is no agreed upon set of criteria for active surveillance, and the large number of criteria out there vary significantly.[6]
Active surveillance
The Klotz criteria for active surveillance - pathologic factors only:[6][7]
Clinical criteria:
- PSA <= 10 ng/mL.[6]
Gross
- Prostate cancer is uncommonly apparent on gross.
- Classic location: posterior aspect of the prostate.
Radiology
- Hypoechoic areas = suspicious for cancer.
Prostatectomy grossing
Cytoprostatectomy grossing
- Limited sampling of the prostate may lead to undersampling error.[14]
Microscopic
Criteria as a list
Major criteria (the ABCs of prostate pathology):[15]
- Architecture.
- Increased gland density.
- Small circular glands.
- In rare subtypes - large branching glands.
- "Infiltrative growth" pattern - malignant glands between benign ones.
- Basal cells lacking.
- Cytological abnormalities:
- Nuclear enlargement (subtle).
- Nucleoli (prominent).
Minor criteria:[15]
- Nuclear hyperchromasia.
- Wispy blue mucin.
- Image: Wispy blue mucin (nature.com) - from Epstein.[16]
- Pink amorphous secretions.
- Image: Pink amorphous secretions (nature.com) - from Epstein.[16]
- Intraluminal crystalloid.
- Image: Intraluminal crystalloid (nature.com) - from Epstein.[16]
- Amphophilic cytoplasm.
- Amphopilic is said to be bluish-red[17] -- though might also be described as blue-grey.
- Adjacent HGPIN.
- Mitoses - quite rare.
Extent/quantity criteria:
- There is no agreed upon minimum number of glands; however, one paper suggests that agreement among experts is low with 5 or less glands.[18]
- Thus, it has been suggested that six or more glands should be present to diagnose cancer.[18]
Pathognomonic features of prostate carcinoma:[19]
- Perineural invasion.
- Must be circumferential (>95% of circumference[citation needed]).
- Glomeruloid bodies.
- Collagenous micronodules.
Divided into high and low power
Low power features
- Architecture is the key to diagnosing low grade cancer.
- Back-to-back glands or crowding of glands -- think low grade cancer (Gleason pattern 3).
- Sharp transition between gland border and lumen.
- Loss of epithelial folding at the epithelium-gland lumen interface - "punched-out" appearance.
- Eosinophilic debris within the gland lumen (pink amorphous secretions, intraluminal crystalloid).
- Blue-tinged acellular material within the gland lumen (mucin) -- uncommon.
- "Infiltrative": small round/oval (malignant) glands (approx. 5 cells across) interspersed with larger (benign) glands that are 2-3 times larger.
High power features
- Nuclei.
- Hyperchromatic nuclei (like in HGPIN).
- Nuclear enlargement, mild (10%?).
- Difficult to appreciate (if cancer isn't side-by-side with normal prostate).
- Difficult/impossible to see at low power.
- Nucleoli visible on high power (200x or 100x magnification).
- May be difficult to see - especially if light intensity is low.
- One should not use 400x to look for nucleoli (it is a waste of time + you risk over-calling something benign).
- May be difficult to see - especially if light intensity is low.
- Loss of basal cells - diagnostic feature.
- Like in breast pathology (where one looks for loss of myoepithelial cells) - this may be difficult to see.
Notes:
- Mitoses are not a common feature.
- If you find them the lesion is probably high-grade.
- Generally, it isn't worth looking for them.
- The nucleoli should be 3 micrometres in size.[citation needed]
Mimics
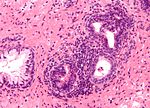
Mimics of prostate adenocarcinoma:[20]
| Entity | Key feature | Detailed microscopic | Other | Image |
|---|---|---|---|---|
| Adenosis (AKA atypical adenomatous hyperplasia) | gradual transition between normal & small gland (NOT two populations) | many small glands, lack nuclear size variation, basal layer present | nucleoli may be present; may need to do p63 or 34betaE12 to find basal layer | AAH (webpathology.com) |
| Sclerosing adenosis | gradual transition between normal & small gland (NOT two populations), fibrosis | many small glands, lack nuclear size variation, basal layer present | analogous to sclerosing adenosis of the breast[citation needed] | Sclerosing adenosis (webpathology.com) |
| Atrophy | sharp angulation of gland | nuclear hyperchromasia, scant cytoplasm | may appear right beside non-atrophic tissue | |
| Basal cell hyperplasia | two distinct cell populations (in epithelial component) | abundant epithelial cells; nucleoli in pale ('blue') nuclei of basal cells, glandular cell nuclei darker ('purple') | vaguely similar to epithelial hyperplasia of usual type (EHUT) in breast | |
| Bulbourethral gland | no nuclear atypia | clear cytoplasm | apex of prostate | |
| Seminal vesicles / ejaculatory ducts | lipofuscin (yellow granular material in cytoplasm), smudge cells (smeared appearance + hyperchromatic) | fern-like arrangement of epithelium (low power), nucleoli, surrounded by muscle, +/- nuclear inclusions | involvement by cancer changes staging, lipofuscin may be present in prostate, often has marked nuc. size var.; location: usu. base of prostate | |
| Radiation effect | marked nuclear size variation | increased stroma (fibrosis), lack nucleoli ??? | history of Rx; uniform nuc. size with Hx of Rx should raise susp. of cancer | Radiation changes (webpathology.com), Radiation changes (webpathology.com) |
| Prostatitis | inflammatory cells (lymphocytes, plasma cells, PMNs) | no nuclear atypia, normal gland arch. | clinical mimic of cancer (elevated PSA); usu. not a problem for the pathologist | |
| Vasitis nodosa | sperm within ducts, clinical history (usu. post-vasectomy) | small tubules, nucleoli common, mild atypia, may "invade" vessels, track along nerves | mimics metastatic prostate carcinoma, IHC stains: PSA-, PSAP- |
Memory device: AAABBRS = atrophy, adenosis, adenosis (sclerosing), basal cell hyperplasia, bulbourethral gland, radiation, seminal vesicles.
Gleason grading system
Overview
- This system is only one any one talks about and there is consensus on how it is done.[21]
- Score range: 6-10.
- Technically 2-10... but almost no one uses 2-5.
- Reported on biopsy (and TURP) as: (primary pattern) + (secondary pattern or tertiary pattern with the highest grade) = sum.
- e.g. Gleason score 3+4=7 means: pattern 3 is present and dominant, pattern 4 is the remainder of the tumour - but present in a lesser amount than pattern 3.
- Reported as on prostatectomies as: (primary pattern) + (secondary pattern) = sum, (tertiary pattern)
- Tertiary Gleason pattern - definition: a pattern that is seen in than 5% of the tumour (volume), that is higher grade than the two dominant patterns.[22]
- The presence of a tertiary patterns adversely affect the prognosis; however, the prognosis is not as bad as when the tertiary pattern is the secondary pattern, i.e. 3+4 tertiary 5 has a better prognosis than 3+5 (with some small amount of pattern 4).[22]
Testing yourself:
- There is a nice test-yourself quiz from Johns Hopkins: http://162.129.103.34/prostate/.
- It was studied in a paper by Kronz et al.[23]
Examples
- A biopsy with 80% pattern 4, 16% pattern 3 and 4% pattern 5... would be reported as: 4+5=9.
- A biopsy with 92% pattern 4, and 8% pattern 3... would be reported as: 4+3=7.
- A biopsy with 98% pattern 4, and 2% pattern 3... would be reported as: 4+4=8.
- A prostatectomy with 80% pattern 4, 16% pattern 3 and 4% pattern 5... would be reported as: 4+3=7 with tertiary pattern 5.
Gleason pattern 1 & 2
- Use strongly discouraged by a number of GU pathology experts.
Notes:
- Gleason pattern 1 - probably represents what today would be called adenosis.
- Should never be used.
- Gleason pattern 2 - used by few GU pathology experts occasionally.
- Generally, should not be diagnosed on core biopsies.[24]
Gleason pattern 3
- Glands smaller than normal prostate glands + loss of epithelial folding.
- Can draw a line around each gland.
- May have gland branching.
- Glands have a X, U, V or Y shape.
Notes:
- Gland lumina should be seen.
- All cribriform is now, generally, classified as Gleason pattern 4.[24]
Gleason pattern 4
- Loss of gland lumina.
- Gland fusion.
- Benign looking cords ('hypernephroid pattern').
- Cribriform.
- Glomeruloid pattern - resembles a glomerulus.
Notes:
- One gland is not enough to call Gleason 4.
Images
www:
Gleason pattern 5
- Sheets.
- Must be differentiated from intraductal growth (which like in the breast are well circumscribed nests).
- Single cells.
- May be confused with stromal/lymphocytic infiltration.
- Look for nucleoli, cells should be round (prostatic stroma cells are spindle cells).
- May be confused with stromal/lymphocytic infiltration.
- Cords (strands).
- Line of cells.
- Should not be intermixed with clumps of cells (pattern 4).
- Nests of cells with necrosis (at the centre) (comedonecrosis) or (intraluminal) necrosis in a cribriform pattern.[21]
- Necrosis:
- Nuclear changes:
- Karyorrhexis (nuclear fragmentation).
- Pynosis (nuclear shrinkage).
- Karyolysis (nuclear dissolution).
- Cell ghosts (cells without a nucleus).
- Nuclear changes:
- Necrosis:
Notes:
- Pattern 5 may be under-diagnosed.
- Single cells is the most commonly missed pattern.[25]
Images
www:
Special types
Special types of prostate cancer have suggested Gleason patterns:[26]
| Special type | Gleason pattern | Comment |
|---|---|---|
| Ductal carcinoma | 4 | may be graded 3 or 5[27] |
| Mucinous carcinoma | dependent on morphology[28][29] | may be graded 4[26] |
| Sarcomatoid carcinoma | 5 | glands graded separately |
| Signet ring cell carcinoma | 5 | |
| Small cell carcinoma | not graded | may be graded 5[27] |
| Adenosquamous and squamous carcinoma | not graded | |
| Lymphoepithelioma-like carcinoma | not graded | |
| Adenoid cystic carcinoma | not graded | |
| Urothelial carcinoma | not graded | |
| Undifferentiated carcinoma, NOS | not graded |
How to remember the ones that aren't graded - think of Ur Lung carcinomas (Urothelial carcinoma, Lymphoepithelioma-like carcinoma):
- Small cell carcinoma.
- Squamous cell carcinoma.
- Adenosquamous carcinoma.
- Adenoid cystic carcinoma.
Biopsy-prostatectomy concordance of Gleason score
- Discordance is common.
- Upgrade on prostatectomy: 25-40%.
- Downgrade on prostatectomy: 5-15%.
Selected studies on concordance:
| Study | Upgrade | Downgrade | Notes |
|---|---|---|---|
| Sfoungaristos et al.[30] | 42.1% | 13.7% | high volume of tumour predicts upgrade |
| Thomas et al.[31] | 38.1% | 4.7% | |
| Truesdale et al.[32] | 23% | 11% |
Sign out
Upgrading
Gleason score upgrading on prostatectomy is considered relatively common; it is reported to occur in 23% to 42.1% of cases.[1][2] 1. BJU Int. 2011 107 (5): 749-54. 2. Can Urol Assoc J. 2012 Jan 24:1-5.
Downgrading
Gleason score downgrading on prostatectomy is considered uncommon; however, it is reported in 4.7% to 13.7% of cases.[1][2] 1. BJU Int. 2012 Jan; 109(2):214-9. 2. Can Urol Assoc J. 2012 Jan; 24;1-5.
Order of Gleason score components changed
COMMENT: The change in the order of the Gleason score components (3+4 on prostatectomy versus 4+3 on core biopsy) may be explained by sampling.
Staging parameters, margins and more
Surgical margins
- Positive is tumour touching ink.† [33]
- "Close" margins (<0.1 mm) have an increased recurrence risk.[33]
Notes:
- Surgical margin - where the surgeon cut.
- It is possible to have EPE without a positive margin.
- It is possible to have a positive margin without EPE.
- † Epstein says not touching may be enough, as tumour close to the margin is damaged from the surgery.[34]
Rates and implication
Positivity rate varies substantially (13-44%):
- Norway: 26% -- strong dependence on surgeon volume (18% high case load vs. 44% low case load).[35]
- France: 13-17% -- PSA and prostate size predictors of positivity.[36]
Note:
- Stage and grade (Gleason score) seem to have less impact than surgeons volume on margin positivity rate.[35]
The impact of positive margins:
- Significant modest negative affect on long-term outcome in node negative cancers (pT2-4 pN0).[37]
- Weaker impact than stage and Gleason score.[38]
- Bladder neck margin positivity may change the T-stage - see below.
Bladder neck margin
- Bladder neck margin positivity typically is pT3a.[40]
- Seen in approximately 1% of prostatectomies.[39]
Extraprostatic extension
- Abbreviated EPE.
General
- Extraprostatic extension (EPE) is difficult to assess in prostatectomy specimens.[41]
- The prostate does NOT have a well defined capsule.
- Intraobserver agreement for EPE is fair-moderate and lower than for the surgical margin.[42]
- The prostate does NOT have a well defined capsule.
- EPE, typically, upstages tumours from T2x to T3a.
Prostatectomy specimens
EPE is present in a prostatectomy if there is either:
- A "significant bulge" in the contour of the prostate at low power and no fibromuscular tissue surrounding the malignant cells.
- Malignant cells directly adjacent to peri-prostatic adipose tissue.
Note:
- The apex of the prostate gland may have some skeletal muscle. Thus, it is difficult to define extension at this site. EPE is not called at the apex by some pathologists; however, it is generally believed to exist.[41]
Prostate biopsy
EPE is present in prostate biopsy if:
- Tumour touches adipose tissue.[43]
Images
Seminal vesicle invasion
- Abbreviated SVI.
General:
- Typically upstages to pT3b.
- Associations:[44]
- Most SVI is by direct extension ~90%.
- Approximately 20% of patients with pT3x have SVI.
- Usually associated with a large tumour volume (22% versus 12%).
Microscopic:
- Tumour must be in the muscle surrounding the epithelial component; tumour in the adventitia (the loose connective tissue surrounding the seminal vesicles) does not count.[45][46]
Notes:
- Invasion of the adventitia (only) would quality as EPE; this is, usually, T3a.
- Immunostains useful to separate prostate carcinoma from SV are discussed in the seminal vesicle article.
- It is not possible to differentiate the seminal vesicles and ejaculatory ducts based only on histology; thus, on biopsy one can generally comment only on seminal vesicle/ejaculatory duct invasion.
Lymph node metastases
- Essentially never happens in Gleason score 6 cancers.
- A study of over 14,000 Gleason score <=6 cases found 22 cases with lymph node metastases -- all of the 19 cases available for review were determined to have a higher Gleason score and some Gleason pattern 4 or 5.[47]
Perineural invasion
- Not a staging parameter.
- Seen in approximately 20% of core biopsies.[48]
- Complete wrapping of a nerve by epithelium is considered pathognomonic for cancer.[49][48]
Note:
- Occasionally, benign glands are found perineural.[48]
- These should not completely wrap around the nerve and should be cytologically benign.
IHC
General recommendations
ISUP consensus statement:[50]
- Should not be used if cancer is obvious.
- Should not be used if it isn't going change the clinical management.
Prostate markers
- PSA (prostate specific antigen) +ve.
- PSAP (prostatic specific acid phosphatase) +ve. †
- P501S +ve. ‡
- NKX3.1 +ve. ‡
Notes:
- † PSAP may be positive in hindgut neuroendocrine tumours.[51]
- ‡ P501S and NKX3.1 are considered second line markers.[50]
- Prostate carcinoma is typically CK7 -ve and CK20 -ve; however, in high Gleason score cancers focal positivity of these markers can be seen.[52]
- CK7: >25-50% staining seen in ~5% of cases.
- >50% staining with CK7 is not report.
- CK20: >25-50% staining seen in ~10% of cases.
- >50% staining with CK20 is not reported.
- CK7: >25-50% staining seen in ~5% of cases.
Benign prostate versus neoplastic prostate
- AMACR +ve.
- p63 -ve.
- HMWCK (34betaE12) -ve.
Combination immunostains:
Other IHC stains:
- AR +ve -- in prostate confined cancer.
- Usu. -ve in LN +ve disease.[56]
Prostate carcinoma versus urothelial carcinoma
The ISUP panel recommends:[50]
- PSA +ve (-ve in UCC).
- GATA3 -ve (+ve in UCC).
Another panel - if GATA3 isn't available:
- Prostate: PSA +ve, p63 -ve, HWMCK -ve.
- Urothelial: p63 +ve, HWMCK +ve, PSA -ve.
Notes:
- AMACR not useful; it is positive in ~50% of UCC.[57]
- CK7 and CK20 are typically negative in prostate carcinoma, and classically positive in urothelial carcinoma.
Rate of utilization
- Dependent on practise setting.
- One tertiary academic institution uses it on ~ 40% of cases.[58]
Molecular changes in prostate cancer
A fusion gene between TMPRSS2 and ERG is described.[59][60]
- Both genes are on chromosome 21.
- Currently not used diagnostically.
- Fusion gene seen in approximately 50% of prostate cancer.[60]
- A subset of TMPRSS2-ERG known as 2+Edel (seen in ~7% of all prostate cancer cases) predicts poor survival.[61]
Sign out
Prostatectomy specimens
- A prostatectomy that appears to be negative should be worked-up. This is discuss in the negative prostatectomy article.
- CAP checklist.
A. LYMPH NODES, RIGHT PELVIC, EXCISION: - ONE LYMPH NODE NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 1 ). B. LYMPH NODES, LEFT PELVIC, EXCISION: - ONE LYMPH NODE NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 1 ). C. PROSTATE GLAND AND SEMINAL VESICLES, RADICAL PROSTATECTOMY: - ADENOCARCINOMA, GLEASON SCORE 7/10 (3+4), pT2c pN0. -- SURGICAL MARGINS NEGATIVE. -- PLEASE SEE TUMOUR SUMMARY.
Transurethral resection of prostate
PROSTATE TISSUE, TRANSURETHRAL RESECTION OF PROSTATE (TURP): - ADENOCARCINOMA, GLEASON SCORE 7/10 (3+4); - APPROXIMATELY 5% OF TISSUE INVOLVED; - PLEASE SEE TUMOUR SUMMARY. TUMOUR SUMMARY - TRANSURETHRAL RESECTION OF PROSTATE (TURP). PROCEDURE: TRANSURETHRAL PROSTATIC RESECTION. SPECIMEN SIZE: WEIGHT: 10 GRAMS. HISTOLOGIC TYPE: ADENOCARCINOMA (ACINAR, NOT OTHERWISE SPECIFIED). HISTOLOGIC GRADING: PRIMARY PATTERN: 3. SECONDARY PATTERN: 4 (40% OF TUMOUR). TOTAL GLEASON SCORE: 7 (3+4). TUMOUR QUANTITATION - PERCENTAGE OF PROSTATIC TISSUE INVOLVED BY TUMOUR: 80 %. PERIPROSTATIC FAT INVASION: NOT IDENTIFIED. SEMINAL VESICLE INVASION: NOT IDENTIFIED. LYMPH-VASCULAR INVASION: NOT IDENTIFIED. PERINEURAL INVASION: NOT IDENTIFIED. ADDITIONAL PATHOLOGIC FINDINGS: HIGH-GRADE PROSTATIC INTRAEPITHELIAL NEOPLASIA (HGPIN). NODULAR PROSTATIC HYPERPLASIA. CHRONIC INFLAMMATION.
PROSTATE TISSUE, TRANSURETHRAL RESECTION OF THE PROSTATE (TURP): - ADENOCARCINOMA, GLEASON SCORE 6/10 (3+3); - APPROXIMATELY 5% OF TISSUE INVOLVED; - PLEASE SEE TUMOUR SUMMARY.
Biopsy specimens
Important elements - a list:[15]
- Type of cancer, e.g. "prostatic adenocarcinoma, acinar type".
- Gleason score including primary and secondary pattern, e.g. "Gleason score 3+4=7".
- Number of cores and number involved, e.g. "2/3 cores involved by cancer".
- Percent area involved, i.e. how much of the core is cancer, e.g. "75% of specimen is tumour". ‡
- Percent area involved that is Gleason pattern 4 or 5, e.g. "25% of the tumour is Gleason pattern 4 or 5".
- Presence of perineural invasion.
- Presence of extension into fat (extraprostatic extension).
Notes:
- ‡ "Percent area involved" may seem like an odd thing to request 'cause it is sampling dependent, i.e. if the radiologist sticks the biopsy needle deeper into the lesion more of the core is positive, but urologists think it is important -- more important than perineural invasion.[62]
- There is disagreement on how one should measure patchy cancer (cancer when there is interspersed normal). Epstein believes one should include the interspersed benign if the cancer is patchy, as the the groupings of tumour likely join out of the plane of section.[63]
- A review by Epstein on the topic of tumour volume suggests it does not have predictive value in multivariante analyses.[63]
- The biopsy tumour volume is a predictor of Gleason score upgrading on prostatectomy.[64]
Completely negative
A. PROSTATE, RIGHT LATERAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. B. PROSTATE, RIGHT MEDIAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. C. PROSTATE, RIGHT LATERAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE. D. PROSTATE, RIGHT MEDIAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE. E. PROSTATE, RIGHT LATERAL INTERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. F. PROSTATE, RIGHT MEDIAL INFERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. G. PROSTATE, LEFT LATERAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. H. PROSTATE, LEFT MEDIAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. I. PROSTATE, LEFT LATERAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE. J. PROSTATE, LEFT MEDIAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE. K. PROSTATE, LEFT LATERAL INTERIOR, BIOPSY: - BENIGN PROSTATE TISSUE. L. PROSTATE, LEFT MEDIAL INFERIOR, BIOPSY: - BENIGN PROSTATE TISSUE.
Negative biopsy in surveillance
COMMENT: The previous results are noted. The absence of cancer in this biopsy may be due to sampling.
No glands
F. PROSTATE, RIGHT MEDIAL MIDZONE, BIOPSY: - BENIGN FIBROMUSCULAR TISSUE; - NO PROSTATIC GLANDULAR TISSUE PRESENT.
Inflammation
G. PROSTATE, LEFT LATERAL SUPERIOR, BIOPSY: - BENIGN PROSTATE TISSUE; - FOCAL CHRONIC INFLAMMATION.
F. PROSTATE, RIGHT MEDIAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE; - CHRONIC INFLAMMATION.
F. PROSTATE, RIGHT MEDIAL MIDZONE, BIOPSY: - BENIGN PROSTATE TISSUE; - ACUTE AND CHRONIC INFLAMMATION.
Positive
F. PROSTATE, RIGHT MEDIAL INFERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 6/10 (3+3); - 1/1 CORE INVOLVED; APPROXIMATELY 5% OF TISSUE INVOLVED.
F. PROSTATE, RIGHT MEDIAL INFERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 6/10 (3+3); - 1/1 CORE INVOLVED; APPROXIMATELY 25% OF TISSUE INVOLVED; - PERINEURAL INVASION PRESENT.
G. PROSTATE, LEFT LATERAL SUPERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 7/10 (4+3); - 1/1 CORE INVOLVED; APPROXIMATELY 5% OF TISSUE INVOLVED; - PERINEURAL INVASION PRESENT.
H. PROSTATE, LEFT MEDIAL SUPERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 8/10 (4+4); - 1/1 CORE INVOLVED; APPROXIMATELY 15% OF TISSUE INVOLVED.
H. PROSTATE, LEFT MEDIAL SUPERIOR, BIOPSY: - ADENOCARCINOMA, GLEASON SCORE 8/10 (4+4); - 1/1 CORE INVOLVED; APPROXIMATELY 15% OF TISSUE INVOLVED; - PERINEURAL INVASION PRESENT.
Tumour summaries
- These are not completely without controversy.
- It should be noted that treatment is driven by the highest Gleason score.[citation needed]
TUMOUR SUMMARY - PROSTATE CORE BIOPSIES: - HISTOLOGIC TYPE: ADENOCARCINOMA (ACINAR, NOT OTHERWISE SPECIFIED). - TOTAL GLEASON SCORE: 7. - PRIMARY PATTERN: 4. - SECONDARY PATTERN: 3. - PERCENT OF TUMOUR WITH PATTERN HIGHER THAN GRADE 3: 75%. - NUMBER OF CORES POSITIVE: 10. - TOTAL NUMBER OF CORES: 12. - TOTAL LINEAR MILLIMETERS OF NEEDLE CORE TISSUE: 152 MM. - PERCENT OF NEEDLE CORE TISSUE THAT IS TUMOUR: 44%. - PERIPROSTATIC FAT INVASION: NOT IDENTIFIED. - SEMINAL VESICLE INVASION: SEMINAL VESICLE NOT IDENTIFIED. - LYMPHOVASCULAR INVASION: NOT IDENTIFIED. - PERINEURAL INVASION: PRESENT. - ADDITIONAL FINDINGS: HIGH-GRADE PROSTATIC INTRAEPITHELIAL NEOPLASIA, CHRONIC INFLAMMATION (FOCAL).
TUMOUR SUMMARY - PROSTATE CORE BIOPSIES: - HISTOLOGIC TYPE: ADENOCARCINOMA (ACINAR, NOT OTHERWISE SPECIFIED). - HIGHEST GLEASON SCORE: 8 (4+4). - SUMMARY GLEASON SCORE: 7 (4+3). - PERCENT OF TUMOUR WITH PATTERN 4: 55%. - PERCENT OF TUMOUR WITH PATTERN 5: 0%. - NUMBER OF CORES POSITIVE: 12. - TOTAL NUMBER OF CORES: 12. - TOTAL LINEAR MILLIMETERS OF NEEDLE CORE TISSUE: 178 MM. - PERCENT OF NEEDLE CORE TISSUE THAT IS TUMOUR: 80%. - PERINEURAL INVASION: PRESENT. - PERIPROSTATIC FAT INVASION: PRESENT. - LYMPHOVASCULAR INVASION: NOT IDENTIFIED. - SEMINAL VESICLE INVASION: NOT IDENTIFIED.
Seminal vesicle/ejaculatory duct invasion on biopsy
COMMENT: The seminal vesicles and ejaculatory ducts have the same histology; thus, it is not usually possible to confidently differentiate them in a needle biopsy. SV/ED invasion was demonstrated with CK7, CK34betaE12/AMACR, PSA and p63 immunostaining. The tumour is PSA and AMACR positive.
Intraductal spread of prostate cancer
Intraductal carcinoma of the prostate
- AKA intraductal carcinoma.
- AKA intraductal prostate carcinoma.
General
- Associated with a poor prognosis.[65]
- Strong association with aggressive invasive carcinomas on prostatectomy when identified in isolation on biopsy.[66]
Microscopic
Major criteria
Required major criteria:[67][68]
- Glands 2x normal (peripheral zone) glands.
- Basal cells present (proven by IHC).
- "Cytologically malignant cells" = nuclear hyperchromasia, nuclear enlargement, nucleoli.
- Fills the lumen ("expansile") but does not have to be "solid".
- Solid = no spaces between the cells.
Additional (major) criterion:[67]
- Comedonecrosis.
Minor criteria
Minor criteria:[67]
- Branching of ducts at right angles.
- Rounded/smooth gland outlines.
- Two cell populations:
- Malignant population (enlarged nuclei with hyperchromasia and nucleoli) = peripheral location in gland.
- Benign population (smaller nuclei, no nucleoli) = central location in gland.
DDx:
- High-grade prostatic intraepithelial neoplasia (HGPIN).
- Invasive prostate adenocarcinoma.
IHC
Features - basal cells present:
- CK34betaE12 +ve.
- p63 +ve.
Unusual forms of prostate cancer
Prostatic ductal adenocarcinoma
General
- Sometimes it is referred to as endometrioid or endometrial adenocarcinoma; both terms are discouraged.[69]
- Not completely uncontroversial - may represent acinar adenocarcinoma with periurethral ducts involvement.[70]
- More aggressive than conventional (acinar) prostate carcinoma.
Microscopic
Features:[71]
- Pseudostratified (crowded appearing) columnar (or cigar-shaped) nuclei - key feature.
- Vaguely resembles colonic adenocarcinoma.
- Compatible architecture:
- Papillary.
- Cribriform.
- Single gland (large glands).
- Endometrioid - vaguely looks like endometrioid endometrial carcinoma (with back-to-back glands).
- >= 50% of tumour.[72][citation needed]
- If ductal component <50%, it is a conventional (acinar) adenocarcinoma with a ductal component.
Notes:
- Proportion of ductal component should be quantified:
- <10% ductal component of no prognostic significance.[72]
Images:
- Prostatic ductal adenocarcinoma - several images (upmc.edu).
- Prostatic ductal adenocarcinoma - another case - several images (upmc.edu).
- Prostatic ductal adenocarcinoma - F1 (nih.gov).
- Prostatic ductal adenocarcinoma - F2 (nih.gov).
- Prostatic ductal adenocarcinoma (webpathology.com).
- Prostatic ductal adenocarcinoma (webpathology.com).
IHC
Features:[73]
- p53 +ve in ~ 75% of cases.
- Ki-67 high in ~ 70% of cases.
- Chromogranin A +ve (cytoplasm) in ~ 70% of cases.
Others:[74]
- PSA +ve.
PIN-like prostatic ductal adenocarcinoma
General
- Recently described.[75][76]
- May be confused with prostatic intraepithelial neoplasia (PIN).
Microscopic
Features:[75]
- Stratified malignant epithelium.
Note:
- Vaguely similar to a tubular adenoma of the colon.
DDx:
Image:
Foamy gland carcinoma
Atrophic prostate carcinoma
- AKA atrophic carcinoma.
General
- Uncommon.
Note:
- An atrophic component in prostate cancer is common; one study identified it in ~15% of cases.[78]
Microscopic
Features:
- Scant cytoplasm.
- Nuclear features of conventional prostate cancer (nucleoli, nuclear enlargement).
- Increased gland density.
DDx:
Image: Atrophic carcinoma (nature.com).
Mucinous prostate carcinoma
General
- Rare.
- Most often Gleason 3+4 ~ 80% in one series of 47 cases.[29]
Gleason pattern:
- In the past, it has been suggested that the mucinous component be assigned Gleason pattern 4.[26]
- The prognosis is similar or may be better than the conventional type of prostate cancer in a large series;[29] thus, it seems reasonable to grade based on the pattern (as advocated by some experts[28]).
Microscopic
Features:
- Cytologically malignant cells floating in mucin.
- > 25% of tumour mucinous.[26]
Notes:
- Mucinous carcinoma - percentage required to call varies by site.
DDx:
- Metastatic mucinous carcinoma.
- Mucinous adenocarcinoma of the prostatic urethra - analogous to the mucinous adenocarcinoma of the urinary bladder.[80]
Pseudohyperplastic prostatic adenocarcinoma
- AKA pseudohyperplastic adenocarcinoma.
General
- Rare.
Microscopic
- Medium to large glands with an atypical morphology - key low power feature:
- Papillary or pseudopapillary infoldings, luminal undulations, branching or cystic dilatation.
- Nuclear features of conventional prostate cancer (nucleoli, nuclear enlargement).
Images
- Pseudohyperplastic adenocarcinoma (webpathology.com).
- Pseudohyperplastic prostatic adenocarcinoma (nature.com).
Notes:
- Usually associated with conventional (acinar) prostate adenocarcinoma.
- Pale abundant cytoplasm - similar to normal prostate.
Prostatic signet ring cell carcinoma
General
- Very rare - 9 cases in a series of 29,783 prostate cancer cases.[83]
- Criteria vary - percentage of SRCs required for Dx varies from 20% to 50%.[83]
Microscopic
Features:
- Signet ring cells - see basics article.
DDx:
- Acinar adenocarcinoma - Gleason pattern 4 with very small glands.
Images
- Prostatic SRCC (nih.gov).
- Prostatic SRCC (nature.com).
- Prostatic SRCC (nature.com).
- Prostatic SRCC (webpathology.com) - looks like acinar adenocarcinoma.
Stains
- Alcian blue-PAS stain +ve.
- PAS -- 50% of cases +ve.[83]
- Alcian blue -- 44% of cases +ve.[83]
Sarcomatoid prostate carcinoma
- AKA carcinosarcoma.
General
- Rare.
Microscopic
Features:[84]
- Biphasic tumour:
- Spindle cells (sarcomatous component).
- May include components of: osteosarcoma, chondrosarcoma and/or rhabdomyosarcoma.
- Glandular component (like conventional prostate carcinoma).
- Spindle cells (sarcomatous component).
IHC
Features - typical:[84]
- PSA +ve.
- Keratin +ve.
Small cell carcinoma of the prostate gland
General
Microscopic
Features:
- Small cells with:
- Nuclear moulding.
- Stippled chromatin.
- High NC ratio.
- +/-High-grade acinar adenocarcinoma, i.e. conventional prostate carcinoma, seen in ~50% of cases.[85]
Notes:
- Similar to small cell carcinoma of the lung.
- High-grade squamoid component favours metastatic urothelial carcinoma.
- UCC usu. HWCK +ve.
DDx:
- Small cell carcinoma of the urinary bladder.
- Lymphoma, large cell.
- Other small round cell tumours.
Images:
- SmCC of the prostate - low mag. (webpathology.com).
- SmCC of the prostate - high mag. (webpathology.com).
- SmCC of the prostate - low mag. (nih.gov).
- SmCC of the prostate - high mag. (nih.gov).
IHC
Features:[85]
- PSA weak +ve/-ve.
- Chromogranin +ve.
Adenoid cystic/basal cell carcinoma of the prostate
- Abbreviated ACBCC.
General
- Rare.
- Typically indolent - may be aggressive.[86]
Microscopic
Features:
- Adenoid cystic carcinoma-like and basal cell adenoma-like:
- Nests of cells that have round spaces filled by whispy blue mucin.
- Dense collagenous stroma.
IHC
- HER2/neu +ve (strong).[87]
Metastatic disease and other cancers of the prostate
Urothelial carcinoma
- Prostatic urothelial carcinoma redirects here.
General
- Spreads from the urinary bladder usually - common.[88]
- Identified by endoscopic loop biopsy.[89]
Treatment:[89]
- Cytoprostatectomy - stromal invasion or extensive intraductal involvement.
- Endoscopic resection and BCG - limited extent without stromal invasion.
Microscopic
Features:
- Divided into tumours with:
- Stromal invasion.
- Without stromal invasion.
Notes:
- Stromal involvement common ~ 75% of cases.[90]
Sign out
PROSTATE TISSUE, TRANSURETHRAL RESECTION: - HIGH-GRADE UROTHELIAL CARCINOMA WITH FOCAL STROMAL INVASION, AND EXTENSIVE INTRADUCTAL SPREAD IN FRAGMENTS WITH BENIGN PROSTATIC GLANDS.
See also
References
- ↑ Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD (August 1993). "The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients". J. Urol. 150 (2 Pt 1): 379–85. PMID 8326560.
- ↑ Li, D.; Kumaraswamy, E.; Harlan-Williams, LM.; Jensen, RA. (2013). "The role of BRCA1 and BRCA2 in prostate cancer.". Front Biosci (Landmark Ed) 18: 1445-59. PMID 23747895.
- ↑ Castro, E.; Eeles, R. (May 2012). "The role of BRCA1 and BRCA2 in prostate cancer.". Asian J Androl 14 (3): 409-14. doi:10.1038/aja.2011.150. PMID 22522501.
- ↑ Taherian, N.; Hamel, N.; Bégin, LR.; Bismar, TA.; Goldgar, DE.; Feng, BJ.; Foulkes, WD. (Feb 2013). "Familial prostate cancer: the damage done and lessons learnt.". Nat Rev Urol 10 (2): 116-22. doi:10.1038/nrurol.2012.257. PMID 23318356.
- ↑ Ip, S.; Dahabreh, IJ.; Chung, M.; Yu, WW.; Balk, EM.; Iovin, RC.; Mathew, P.; Luongo, T. et al. (Dec 2011). "An evidence review of active surveillance in men with localized prostate cancer.". Evid Rep Technol Assess (Full Rep) (204): 1-341. PMID 23126653. http://www.ncbi.nlm.nih.gov/books/NBK83054/.
- ↑ 6.0 6.1 6.2 Palisaar, JR.; Noldus, J.; Löppenberg, B.; von Bodman, C.; Sommerer, F.; Eggert, T. (Sep 2012). "Comprehensive report on prostate cancer misclassification by 16 currently used low-risk and active surveillance criteria.". BJU Int 110 (6 Pt B): E172-81. doi:10.1111/j.1464-410X.2012.10935.x. PMID 22314081.
- ↑ Klotz, L. (Nov 2005). "Active surveillance for prostate cancer: for whom?". J Clin Oncol 23 (32): 8165-9. doi:10.1200/JCO.2005.03.3134. PMID 16278468.
- ↑ URL: http://www.active-surveillance.com/laurence-klotz-md/. Accessed on: 12 July 2013.
- ↑ Klotz, L. (May 2013). "Active surveillance: patient selection.". Curr Opin Urol 23 (3): 239-44. doi:10.1097/MOU.0b013e32835f8f6b. PMID 23548978.
- ↑ Klotz, L. (2012). "Active surveillance for low-risk prostate cancer.". F1000 Med Rep 4: 16. doi:10.3410/M4-16. PMC 3412317. PMID 22891078. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3412317/.
- ↑ Fleshner, NE.; O'Sullivan, M.; Premdass, C.; Fair, WR. (Feb 1999). "Clinical significance of small (less than 0.2 cm3) hypoechoic lesions in men with normal digital rectal examinations and prostate-specific antigen levels less than 10 ng/mL.". Urology 53 (2): 356-8. PMID 9933054.
- ↑ Nakano Junqueira, VC.; Zogbi, O.; Cologna, A.; Dos Reis, RB.; Tucci, S.; Reis, LO.; Westphalen, AC.; Muglia, VF. (Oct 2012). "Is a visible (hypoechoic) lesion at biopsy an independent predictor of prostate cancer outcome?". Ultrasound Med Biol 38 (10): 1689-94. doi:10.1016/j.ultrasmedbio.2012.06.006. PMID 22920545.
- ↑ Devonec, M.; Fendler, JP.; Monsallier, M.; Mouriquand, P.; Maquet, JH.; Mestas, JL.; Dutrieux-Berger, N.; Perrin, P. (Feb 1990). "The significance of the prostatic hypoechoic area: results in 226 ultrasonically guided prostatic biopsies.". J Urol 143 (2): 316-9. PMID 1688955.
- ↑ Cindolo, L.; Benincasa, G.; Autorino, R.; Domizio, S.; De Rosa, G.; Testa, G.; D'Armiento, M.; Altieri, V.. "Prevalence of silent prostatic adenocarcinoma in 165 patients undergone cystoprostatectomy: a retrospective study.". Oncol Rep 8 (2): 269-71. PMID 11182038.
- ↑ 15.0 15.1 15.2 Humphrey PA (January 2007). "Diagnosis of adenocarcinoma in prostate needle biopsy tissue". J. Clin. Pathol. 60 (1): 35–42. doi:10.1136/jcp.2005.036442. PMC 1860598. PMID 17213347. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860598/?tool=pubmed.
- ↑ 16.0 16.1 16.2 Epstein JI (March 2004). "Diagnosis and reporting of limited adenocarcinoma of the prostate on needle biopsy". Mod. Pathol. 17 (3): 307–15. doi:10.1038/modpathol.3800050. PMID 14739905. http://www.nature.com/modpathol/journal/v17/n3/full/3800050a.html.
- ↑ URL: http://pancreaticcancer2000.com/page1.htm. Accessed on: 3 June 2010.
- ↑ 18.0 18.1 Van der Kwast, TH.; Evans, A.; Lockwood, G.; Tkachuk, D.; Bostwick, DG.; Epstein, JI.; Humphrey, PA.; Montironi, R. et al. (Feb 2010). "Variability in diagnostic opinion among pathologists for single small atypical foci in prostate biopsies.". Am J Surg Pathol 34 (2): 169-77. doi:10.1097/PAS.0b013e3181c7997b. PMID 20061936.
- ↑ Egevad, L.; Allsbrook, WC.; Epstein, JI. (Mar 2006). "Current practice of diagnosis and reporting of prostate cancer on needle biopsy among genitourinary pathologists.". Hum Pathol 37 (3): 292-7. PMID 16613324.
- ↑ Weedman Molavi, Diana (2008). The Practice of Surgical Pathology: A Beginner's Guide to the Diagnostic Process (1st ed.). Springer. pp. 100-3. ISBN 978-0387744858.
- ↑ 21.0 21.1 Epstein, JI.; Allsbrook, WC.; Amin, MB.; Egevad, LL. (Sep 2005). "The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma.". Am J Surg Pathol 29 (9): 1228-42. PMID 16096414.
- ↑ 22.0 22.1 Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 72. ISBN 978-0443066771.
- ↑ Kronz, JD.; Silberman, MA.; Allsbrook, WC.; Bastacky, SI.; Burks, RT.; Cina, SJ.; Mills, SE.; Ross, JS. et al. (Sep 2000). "Pathology residents' use of a Web-based tutorial to improve Gleason grading of prostate carcinoma on needle biopsies.". Hum Pathol 31 (9): 1044-50. doi:10.1053/hupa.2000.16278. PMID 11014569.
- ↑ 24.0 24.1 Epstein, JI. (Feb 2010). "An update of the Gleason grading system.". J Urol 183 (2): 433-40. doi:10.1016/j.juro.2009.10.046. PMID 20006878. Cite error: Invalid
<ref>tag; name "pmid20006878" defined multiple times with different content - ↑ Fajardo, DA.; Miyamoto, H.; Miller, JS.; Lee, TK.; Epstein, JI. (Nov 2011). "Identification of Gleason pattern 5 on prostatic needle core biopsy: frequency of underdiagnosis and relation to morphology.". Am J Surg Pathol 35 (11): 1706-11. doi:10.1097/PAS.0b013e318228571d. PMID 21997691.
- ↑ 26.0 26.1 26.2 26.3 Grignon DJ (March 2004). "Unusual subtypes of prostate cancer". Mod. Pathol. 17 (3): 316–27. doi:10.1038/modpathol.3800052. PMID 14976541.
- ↑ 27.0 27.1 URL: https://www.bostwicklaboratories.com/global/physicians/medical-library/articles/gleason-grading.aspx. Accessed on: 26 November 2011.
- ↑ 28.0 28.1 Osunkoya, AO.; Adsay, NV.; Cohen, C.; Epstein, JI.; Smith, SL. (Jul 2008). "MUC2 expression in primary mucinous and nonmucinous adenocarcinoma of the prostate: an analysis of 50 cases on radical prostatectomy.". Mod Pathol 21 (7): 789-94. doi:10.1038/modpathol.2008.47. PMID 18487999.
- ↑ 29.0 29.1 29.2 Osunkoya, AO.; Nielsen, ME.; Epstein, JI. (Mar 2008). "Prognosis of mucinous adenocarcinoma of the prostate treated by radical prostatectomy: a study of 47 cases.". Am J Surg Pathol 32 (3): 468-72. doi:10.1097/PAS.0b013e3181589f72. PMID 18300802.
- ↑ Sfoungaristos, S.; Perimenis, P. (Jan 2012). "Clinical and pathological variables that predict changes in tumour grade after radical prostatectomy in patients with prostate cancer.". Can Urol Assoc J: 1-5. doi:10.5489/cuaj.11067. PMID 22277633.
- ↑ Thomas, C.; Pfirrmann, K.; Pieles, F.; Bogumil, A.; Gillitzer, R.; Wiesner, C.; Thüroff, JW.; Melchior, SW. (Jan 2012). "Predictors for clinically relevant Gleason score upgrade in patients undergoing radical prostatectomy.". BJU Int 109 (2): 214-9. doi:10.1111/j.1464-410X.2011.10187.x. PMID 21592293.
- ↑ Truesdale, MD.; Cheetham, PJ.; Turk, AT.; Sartori, S.; Hruby, GW.; Dinneen, EP.; Benson, MC.; Badani, KK. (Mar 2011). "Gleason score concordance on biopsy-confirmed prostate cancer: is pathological re-evaluation necessary prior to radical prostatectomy?". BJU Int 107 (5): 749-54. doi:10.1111/j.1464-410X.2010.09570.x. PMID 20840549.
- ↑ 33.0 33.1 Lu, J.; Wirth, GJ.; Wu, S.; Chen, J.; Dahl, DM.; Olumi, AF.; Young, RH.; McDougal, WS. et al. (Jul 2012). "A close surgical margin after radical prostatectomy is an independent predictor of recurrence.". J Urol 188 (1): 91-7. doi:10.1016/j.juro.2012.02.2565. PMID 22578729.
- ↑ URL: http://urology.jhu.edu/newsletter/prostate_cancer410.php. Accessed on: 26 March 2013.
- ↑ 35.0 35.1 Steinsvik, EA.; Axcrona, K.; Angelsen, A.; Beisland, C.; Dahl, A.; Eri, LM.; Haug, ES.; Svindland, A. et al. (Aug 2012). "Does a surgeon's annual radical prostatectomy volume predict the risk of positive surgical margins and urinary incontinence at one-year follow-up? - Findings from a prospective national study.". Scand J Urol Nephrol. doi:10.3109/00365599.2012.707684. PMID 22860630.
- ↑ Koutlidis, N.; Mourey, E.; Champigneulle, J.; Mangin, P.; Cormier, L. (Jul 2012). "Robot-assisted or pure laparoscopic nerve-sparing radical prostatectomy: What is the optimal procedure for the surgical margins? A single center experience.". Int J Urol. doi:10.1111/j.1442-2042.2012.03102.x. PMID 22860572.
- ↑ Mauermann, J.; Fradet, V.; Lacombe, L.; Dujardin, T.; Tiguert, R.; Tetu, B.; Fradet, Y. (Aug 2012). "The Impact of Solitary and Multiple Positive Surgical Margins on Hard Clinical End Points in 1712 Adjuvant Treatment-Naive pT2-4 N0 Radical Prostatectomy Patients.". Eur Urol. doi:10.1016/j.eururo.2012.08.002. PMID 22901983.
- ↑ Chalfin, HJ.; Dinizo, M.; Trock, BJ.; Feng, Z.; Partin, AW.; Walsh, PC.; Humphreys, E.; Han, M. (Jul 2012). "Impact of surgical margin status on prostate-cancer-specific mortality.". BJU Int. doi:10.1111/j.1464-410X.2012.11371.x. PMID 22788795.
- ↑ 39.0 39.1 Pierorazio, PM.; Epstein, JI.; Humphreys, E.; Han, M.; Walsh, PC.; Partin, AW. (Jan 2010). "The significance of a positive bladder neck margin after radical prostatectomy: the American Joint Committee on Cancer Pathological Stage T4 designation is not warranted.". J Urol 183 (1): 151-7. doi:10.1016/j.juro.2009.08.138. PMID 19914651.
- ↑ {{Cite journal | last1 = Chung | first1 = MS. | last2 = Lee | first2 = SH. | last3 = Lee | first3 = DH. | last4 = Chung | first4 = BH. | title = Evaluation of the 7th American Joint Committee on cancer TNM staging system for prostate cancer in point of classification of bladder neck invasion. | journal = Jpn J Clin Oncol | volume = 43 | issue = 2 | pages = 184-8 | month = Feb | year = 2013 | doi = 10.1093/jjco/hys196 | PMID = 23225909 }
- ↑ 41.0 41.1 Magi-Galluzzi, C.; Evans, AJ.; Delahunt, B.; Epstein, JI.; Griffiths, DF.; van der Kwast, TH.; Montironi, R.; Wheeler, TM. et al. (Jan 2011). "International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease.". Mod Pathol 24 (1): 26-38. doi:10.1038/modpathol.2010.158. PMID 20802467.
- ↑ Evans, AJ.; Henry, PC.; Van der Kwast, TH.; Tkachuk, DC.; Watson, K.; Lockwood, GA.; Fleshner, NE.; Cheung, C. et al. (Oct 2008). "Interobserver variability between expert urologic pathologists for extraprostatic extension and surgical margin status in radical prostatectomy specimens.". Am J Surg Pathol 32 (10): 1503-12. doi:10.1097/PAS.0b013e31817fb3a0. PMID 18708939.
- ↑ Epstein, JI.; Srigley, J.; Grignon, D.; Humphrey, P. (Sep 2007). "Recommendations for the reporting of prostate carcinoma.". Hum Pathol 38 (9): 1305-9. doi:10.1016/j.humpath.2007.05.015. PMID 17707261.
- ↑ Sapre, N.; Pedersen, J.; Hong, MK.; Harewood, L.; Peters, J.; Costello, AJ.; Hovens, CM.; Corcoran, NM. (Dec 2012). "Re-evaluating the biological significance of seminal vesicle invasion (SVI) in locally advanced prostate cancer.". BJU Int 110 Suppl 4: 58-63. doi:10.1111/j.1464-410X.2012.11477.x. PMID 23194127.
- ↑ Lester, Susan Carole (2010). Manual of Surgical Pathology (3rd ed.). Saunders. pp. 409. ISBN 978-0-323-06516-0.
- ↑ Berney, DM.; Wheeler, TM.; Grignon, DJ.; Epstein, JI.; Griffiths, DF.; Humphrey, PA.; van der Kwast, T.; Montironi, R. et al. (Jan 2011). "International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 4: seminal vesicles and lymph nodes.". Mod Pathol 24 (1): 39-47. doi:10.1038/modpathol.2010.160. PMID 20818343.
- ↑ Ross, HM.; Kryvenko, ON.; Cowan, JE.; Simko, JP.; Wheeler, TM.; Epstein, JI. (Sep 2012). "Do adenocarcinomas of the prostate with Gleason score (GS) ≤6 have the potential to metastasize to lymph nodes?". Am J Surg Pathol 36 (9): 1346-52. doi:10.1097/PAS.0b013e3182556dcd. PMID 22531173.
- ↑ 48.0 48.1 48.2 Ali, TZ.; Epstein, JI. (Sep 2005). "Perineural involvement by benign prostatic glands on needle biopsy.". Am J Surg Pathol 29 (9): 1159-63. PMID 16096404.
- ↑ Baisden, BL.; Kahane, H.; Epstein, JI. (Aug 1999). "Perineural invasion, mucinous fibroplasia, and glomerulations: diagnostic features of limited cancer on prostate needle biopsy.". Am J Surg Pathol 23 (8): 918-24. PMID 10435561.
- ↑ 50.0 50.1 50.2 Amin MB, Epstein JI, Ulbright TM, et al. (August 2014). "Best practices recommendations in the application of immunohistochemistry in urologic pathology: report from the international society of urological pathology consensus conference". Am. J. Surg. Pathol. 38 (8): 1017–22. doi:10.1097/PAS.0000000000000254. PMID 25025364.
- ↑ Azumi, N.; Traweek, ST.; Battifora, H. (Aug 1991). "Prostatic acid phosphatase in carcinoid tumors. Immunohistochemical and immunoblot studies.". Am J Surg Pathol 15 (8): 785-90. PMID 1712549.
- ↑ Goldstein, NS. (Mar 2002). "Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores.". Am J Clin Pathol 117 (3): 471-7. doi:10.1309/G6PR-Y774-X738-FG2K. PMID 11888088.
- ↑ URL: http://biocare.net/wp-content/uploads/225DS.pdf. Accessed on: 18 October 2011.
- ↑ URL: http://www.antibodies-online.com/antibody/308235/anti-PIN-4+p63+Cytokeratin+HMW+p504S++AMACR/. Accessed on: 18 October 2011.
- ↑ URL: http://www.webpathology.com/image.asp?case=96&n=5. Accessed on: 18 October 2011.
- ↑ Fleischmann, A.; Rocha, C.; Schobinger, S.; Seiler, R.; Wiese, B.; Thalmann, GN. (Apr 2011). "Androgen receptors are differentially expressed in Gleason patterns of prostate cancer and down-regulated in matched lymph node metastases.". Prostate 71 (5): 453-60. doi:10.1002/pros.21259. PMID 20878946.
- ↑ Langner, C.; Rupar, G.; Leibl, S.; Hutterer, G.; Chromecki, T.; Hoefler, G.; Rehak, P.; Zigeuner, R. (Mar 2006). "Alpha-methylacyl-CoA racemase (AMACR/P504S) protein expression in urothelial carcinoma of the upper urinary tract correlates with tumour progression.". Virchows Arch 448 (3): 325-30. doi:10.1007/s00428-005-0129-6. PMID 16315020.
- ↑ Watson, K.; Wang, C.; Yilmaz, A.; Bismar, TA.; Trpkov, K. (Apr 2013). "Use of immunohistochemistry in routine workup of prostate needle biopsies: a tertiary academic institution experience.". Arch Pathol Lab Med 137 (4): 541-5. doi:10.5858/arpa.2012-0145-OA. PMID 23273390.
- ↑ Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, Gong Y, Cheng H, Laxman B, Vellaichamy A, Shankar S, Li Y, Dhanasekaran SM, Morey R, Barrette T, Lonigro RJ, Tomlins SA, Varambally S, Qin ZS, Chinnaiyan AM (May 2010). "An Integrated Network of Androgen Receptor, Polycomb, and TMPRSS2-ERG Gene Fusions in Prostate Cancer Progression". Cancer Cell 17 (5): 443–54. doi:10.1016/j.ccr.2010.03.018. PMC 2874722. PMID 20478527. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2874722/.
- ↑ 60.0 60.1 Online 'Mendelian Inheritance in Man' (OMIM) 602060
- ↑ Attard, G.; Clark, J.; Ambroisine, L.; Fisher, G.; Kovacs, G.; Flohr, P.; Berney, D.; Foster, CS. et al. (Jan 2008). "Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer.". Oncogene 27 (3): 253-63. doi:10.1038/sj.onc.1210640. PMID 17637754.
- ↑ Rubin MA, Bismar TA, Curtis S, Montie JE (July 2004). "Prostate needle biopsy reporting: how are the surgical members of the Society of Urologic Oncology using pathology reports to guide treatment of prostate cancer patients?". Am. J. Surg. Pathol. 28 (7): 946–52. PMID 15223967.
- ↑ 63.0 63.1 Epstein, JI. (Sep 2011). "Prognostic significance of tumor volume in radical prostatectomy and needle biopsy specimens.". J Urol 186 (3): 790-7. doi:10.1016/j.juro.2011.02.2695. PMID 21788055.
- ↑ Fu, Q.; Moul, JW.; Bañez, LL.; Sun, L.; Mouraviev, V.; Xie, D.; Polascik, TJ. (Dec 2012). "Association between percentage of tumor involvement and Gleason score upgrading in low-risk prostate cancer.". Med Oncol 29 (5): 3339-44. doi:10.1007/s12032-012-0270-4. PMID 22688447.
- ↑ Henry, PC.; Evans, AJ. (Jul 2009). "Intraductal carcinoma of the prostate: a distinct histopathological entity with important prognostic implications.". J Clin Pathol 62 (7): 579-83. doi:10.1136/jcp.2009.065003. PMID 19246509.
- ↑ Robinson, BD.; Epstein, JI. (Oct 2010). "Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings.". J Urol 184 (4): 1328-33. doi:10.1016/j.juro.2010.06.017. PMID 20723921.
- ↑ 67.0 67.1 67.2 Shah, RB.; Zhou, M. (Jul 2012). "Atypical cribriform lesions of the prostate: clinical significance, differential diagnosis and current concept of intraductal carcinoma of the prostate.". Adv Anat Pathol 19 (4): 270-8. doi:10.1097/PAP.0b013e31825c6c0e. PMID 22692290.
- ↑ Cohen, RJ.; Wheeler, TM.; Bonkhoff, H.; Rubin, MA. (Jul 2007). "A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma.". Arch Pathol Lab Med 131 (7): 1103-9. doi:10.1043/1543-2165(2007)131[1103:APOTIH]2.0.CO;2. PMID 17616999.
- ↑ Samaratunga, H.; Delahunt, B. (Aug 2008). "Ductal adenocarcinoma of the prostate: current opinion and controversies.". Anal Quant Cytol Histol 30 (4): 237-46. PMID 18773743.
- ↑ Bock, BJ.; Bostwick, DG. (Jul 1999). "Does prostatic ductal adenocarcinoma exist?". Am J Surg Pathol 23 (7): 781-5. PMID 10403300.
- ↑ Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 88. ISBN 978-0443066771.
- ↑ 72.0 72.1 Amin, A.; Epstein, JI. (Apr 2011). "Pathologic stage of prostatic ductal adenocarcinoma at radical prostatectomy: effect of percentage of the ductal component and associated grade of acinar adenocarcinoma.". Am J Surg Pathol 35 (4): 615-9. doi:10.1097/PAS.0b013e31820eb25b. PMID 21383610.
- ↑ Tarján, M.; Lenngren, A.; Hellberg, D.; Tot, T. (Jun 2012). "Immunohistochemical verification of ductal differentiation in prostate cancer.". APMIS 120 (6): 510-8. doi:10.1111/j.1600-0463.2011.02862.x. PMID 22583364.
- ↑ Kumar, A.; Mukherjee, SD. (Apr 2010). "Metastatic ductal carcinoma of the prostate: a rare variant responding to a common treatment.". Can Urol Assoc J 4 (2): E50-4. PMID 20368883.
- ↑ 75.0 75.1 Hameed, O.; Humphrey, PA. (Jul 2006). "Stratified epithelium in prostatic adenocarcinoma: a mimic of high-grade prostatic intraepithelial neoplasia.". Mod Pathol 19 (7): 899-906. doi:10.1038/modpathol.3800601. PMID 16607376.
- ↑ Lee, TK.; Miller, JS.; Epstein, JI. (Jun 2010). "Rare histological patterns of prostatic ductal adenocarcinoma.". Pathology 42 (4): 319-24. doi:10.3109/00313021003767314. PMID 20438402.
- ↑ Zhao, J.; Epstein, JI. (Apr 2009). "High-grade foamy gland prostatic adenocarcinoma on biopsy or transurethral resection: a morphologic study of 55 cases.". Am J Surg Pathol 33 (4): 583-90. doi:10.1097/PAS.0b013e31818a5c6c. PMID 19033862.
- ↑ Kaleem, Z.; Swanson, PE.; Vollmer, RT.; Humphrey, PA. (Jun 1998). "Prostatic adenocarcinoma with atrophic features: a study of 202 consecutive completely embedded radical prostatectomy specimens.". Am J Clin Pathol 109 (6): 695-703. PMID 9620026.
- ↑ Osunkoya AO, Nielsen ME, Epstein JI (March 2008). "Prognosis of mucinous adenocarcinoma of the prostate treated by radical prostatectomy: a study of 47 cases". Am. J. Surg. Pathol. 32 (3): 468–72. doi:10.1097/PAS.0b013e3181589f72. PMID 18300802.
- ↑ 80.0 80.1 Bohman, KD.; Osunkoya, AO. (Nov 2012). "Mucin-producing tumors and tumor-like lesions involving the prostate: a comprehensive review.". Adv Anat Pathol 19 (6): 374-87. doi:10.1097/PAP.0b013e318271a361. PMID 23060063.
- ↑ Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 77. ISBN 978-0443066771.
- ↑ Arista-Nasr J, Martinez-Benitez B, Valdes S, Hernández M, Bornstein-Quevedo L (2003). "Pseudohyperplastic prostatic adenocarcinoma in transurethral resections of the prostate". Pathol. Oncol. Res. 9 (4): 232–5. doi:PAOR.2003.9.4.0232. PMID 14688829.
- ↑ 83.0 83.1 83.2 83.3 Warner, JN.; Nakamura, LY.; Pacelli, A.; Humphreys, MR.; Castle, EP. (Dec 2010). "Primary signet ring cell carcinoma of the prostate.". Mayo Clin Proc 85 (12): 1130-6. doi:10.4065/mcp.2010.0463. PMID 21123640.
- ↑ 84.0 84.1 Zhou, Ming; Magi-Galluzzi, Cristina (2006). Genitourinary Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 77 & 80. ISBN 978-0443066771.
- ↑ 85.0 85.1 85.2 85.3 Furtado, P.; Lima, MV.; Nogueira, C.; Franco, M.; Tavora, F. (2011). "Review of small cell carcinomas of the prostate.". Prostate Cancer 2011: 543272. doi:10.1155/2011/543272. PMID 22110988.
- ↑ Iczkowski, KA.; Ferguson, KL.; Grier, DD.; Hossain, D.; Banerjee, SS.; McNeal, JE.; Bostwick, DG. (Dec 2003). "Adenoid cystic/basal cell carcinoma of the prostate: clinicopathologic findings in 19 cases.". Am J Surg Pathol 27 (12): 1523-9. PMID 14657711.
- ↑ Iczkowski, KA.; Montironi, R. (Dec 2006). "Adenoid cystic/basal cell carcinoma of the prostate strongly expresses HER-2/neu.". J Clin Pathol 59 (12): 1327-30. doi:10.1136/jcp.2005.035147. PMID 17142577.
- ↑ Huguet, J. (Oct 2012). "[Prostatic involvement by urothelial carcinoma in patients with bladder cancer and their implications in the clinical practice].". Actas Urol Esp 36 (9): 545-53. doi:10.1016/j.acuro.2012.02.005. PMID 22520044.
- ↑ 89.0 89.1 Liedberg, F.; Chebil, G.; Månsson, W. (Mar 2007). "Urothelial carcinoma in the prostatic urethra and prostate: current controversies.". Expert Rev Anticancer Ther 7 (3): 383-90. doi:10.1586/14737140.7.3.383. PMID 17338657.
- ↑ Ichihara, K.; Masumori, N.; Kitamura, H.; Hasegawa, T.; Tsukamoto, T. (Dec 2012). "Clinical outcomes of urothelial carcinoma of the prostate detected in radical cystectomy specimens.". Int J Clin Oncol. doi:10.1007/s10147-012-0508-3. PMID 23250619.