Difference between revisions of "Invasive breast cancer"
(→Invasive lobular carcinoma: +images) |
|||
| Line 421: | Line 421: | ||
Features: | Features: | ||
*"Single file" - cell line-up in a row. | *"Single file" - cell line-up in a row. | ||
**Cell should not be cohesive -- lymphoma should briefly come to mind. | **Cell should not be cohesive -- [[lymphoma]] should briefly come to mind. | ||
***primary lymphoma of the breast exists... but it is extremely rare. | ***primary lymphoma of the breast exists... but it is extremely rare. | ||
*NO gland formation. | *NO gland formation. | ||
| Line 429: | Line 429: | ||
Note: | Note: | ||
* | *Commonly have low grade nuclear features. | ||
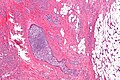
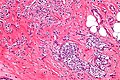
Images | ====Images==== | ||
<gallery> | |||
Image:Lobular_carcinoma_-_low_mag.jpg | Lobular carcinoma - low mag. (WC/Nephron) | |||
Image:Lobular_carcinoma_-_high_mag.jpg | Lobular carcinoma - high mag. (WC/Nephron) | |||
Image:Lobular_carcinoma_-_high_mag.jpg | Lobular carcinoma - very high mag. (WC/Nephron) | |||
</gallery> | |||
More [[WC]] images: | |||
*[http://commons.wikimedia.org/wiki/File:Breast_invasive_lobular_carcinoma_%281%29.jpg Lobular carcinoma - 1 (WC)]. | *[http://commons.wikimedia.org/wiki/File:Breast_invasive_lobular_carcinoma_%281%29.jpg Lobular carcinoma - 1 (WC)]. | ||
*[http://commons.wikimedia.org/wiki/File:Breast_invasive_lobular_carcinoma_%282%29.jpg Lobular carcinoma - 2 (WC)]. | *[http://commons.wikimedia.org/wiki/File:Breast_invasive_lobular_carcinoma_%282%29.jpg Lobular carcinoma - 2 (WC)]. | ||
Subclassification | ====Subclassification==== | ||
*Classic lobular carcinoma. | *Classic lobular carcinoma. | ||
**Low nuclear grade - NO significant variation of nucleus size. | **Low nuclear grade - NO significant variation of nucleus size. | ||
*Pleomorphic lobular carcinoma. | *Pleomorphic lobular carcinoma. | ||
**Significant nuclear atypia. | **Significant nuclear atypia. | ||
Note: Some pathologist grade lobular carcinoma like other types and avoid the term "pleomorphic lobular carcinoma."<ref>MUA. Jan 22, 2009.</ref> | |||
Note: | |||
*Some pathologist grade lobular carcinoma like other types and avoid the term "pleomorphic lobular carcinoma."<ref>MUA. Jan 22, 2009.</ref> | |||
==Medullary breast carcinoma== | ==Medullary breast carcinoma== | ||
Revision as of 02:13, 15 May 2013
The article deals with invasive breast cancer and the evaluation of hormone receptor & HER2 status. Non-invasive breast cancer is dealt with in non-invasive breast cancer.
Introduction
Overview of invasive breast cancer subtypes
Common epithelial subtypes
Type and percentage of breast carcinomas:[1]
- Ductal - AKA no special type (NST) - 79%.
- Lobular - 10%.
- Cribriform / tubular - 6%.
- Mucinous (colloid) - 2%.
- Medullary - 2%.
- Papillary - 1%.
- Metaplastic - <1%.
Common stromal types
- Malignant phyllodes tumour.
- Angiosarcoma - post-radiation ~ 10 years.[2]
Good prognosis subtypes
Three good prognosis subtypes:[3]
- Tubular carcinoma.
- Mucinous carcinoma.
- Papillary carcinoma.
Comprehensive list of invasive breast cancer subtypes
Epithelial
Counterparts of in situ lesions:
- Invasive ductal carinoma, not otherwise specified.
- Invasive lobular carcinoma.
- Invasive cribriform carcinoma.
- Invasive papillary carcinoma.
- Invasive micropapillary carcinoma.
Other epithelial tumours:
- Tubular carcinoma.
- Medullary carcinoma.
- Mucinous carinoma.
- Metaplastic carcinoma.
- Neuroendocrine tumour.
- Apocrine carcinoma.
- Lipid-rich carcinoma.
- Secretory carcinoma.
- Oncocytic carcinoma.
- Glycogen-rich clear cell carcinoma.
Epithelial tumours seen in the salivary gland:
Seen in the skin:
Clinically diagnosed:
- Inflammatory carcinoma.
In situ lesions:
Proliferative lesions:
Non-specific:
- Microinvasive carcinoma.
Papillary:
- Papilloma.
- Atypical papilloma.
- Intraductal papillary carcinoma.
Adenomas:
- Ductal adenoma.
- Tubular adenoma.
- Lactating adenoma.
- Apocrine adenoma.
- Pleomorphic adenoma.
Myoepithelial
- Myoepitheliosis.
- Adenomyoepithelial adenosis.
- Adenomyoepithelioma.
- Malignant adenomyoepithelioma.
Mesenchymal tumours
- See: Soft tissue lesions.
Fibroepithelial tumours
- Fibroadenoma.
- Phyllodes tumour.
- Periductal stromal sarcoma, low grade.
- Mammary hamartoma.
Nipple lesions
- Nipple adenoma.
- Syringomatous adenoma.
- Paget disease of the breast.
Other
Familial breast cancer
Syndromes associated with breast cancer
| Gene | Syndrome | Other cancers | Notes |
|---|---|---|---|
| BRCA1 | Familial breast and ovarian cancer[4] | male breast, ovarian, prostate, pancreas, fallopian tube | younger individuals vis-à-vis BRCA2 |
| BRCA2 | Familial breast and ovarian cancer 2[5] | male breast, ovarian, prostate, pancreas, stomach, melanoma, gallbladder, bile duct, pharynx | older individuals vis-à-vis BRCA1 |
| TP53 (p53) | Li-Fraumeni syndrome (AKA SBLA syndrome) | sarcomas, brain cancer, larynx, lung, leukemia, adrenal cortical carcinoma | often present in childhood |
| CHEK2 | Li-Fraumeni syndrome (variant) | see p53 | - |
| STK11 | Peutz-Jeghers syndrome | breast cancer, GI cancer, Sertoli cell tumour, Granulosa cell tumour, SCTAT | characteristic GI hamartomas, mucocutaneous pigmentation |
| PTEN | Cowden syndrome | breast, thyroid (PTC), endometrial, renal, colorectal | - |
| CDH1 | Familial diffuse gastric cancer[6] | invasive lobular carcinoma, gastric signet ring cell carcinoma | - |
BRCA1 and BRCA2
BRCA1 vs. BRCA2:[7]
| Gene | Age | Histology | Other cancers |
|---|---|---|---|
| BRCA1 | younger | worse types, e.g. triple negative breast ca. | uterine tube |
| BRCA2 | older | sporadic types | stomach, melanoma, gallbladder, bile duct, pharynx |
Types of cancer associated with both BRCA1 and BRCA2 - male OPP:
- Male breast, ovarian, prostate, pancreas.
How to remember types of cancer associated with BRCA2 - PUM:
- Pharynx, upper GI (stomach, gallbladder, biliary), melanoma.
Breast IHC
Molecular classification of invasive carcinoma
A molecular classification:[8]
| Type | Percentage | IHC | Histology | Prognosis/clinical |
|---|---|---|---|---|
| Luminal A | ~45% | ER+ PR+ HER2- | well-differentiated | good, chemo resistant |
| Luminal B | 17% | ER+ PR+ HER2+ | high grade | poor, +/- chemo responsive |
| Normal breast-like | ~8% | ER+ PR+ (?) HER2- | well-differentiated | good |
| Basal-like | ~20% | ER- PR- HER2- | poorly differentiated | aggressive, may have good chemo response, classic for BRCA1 mutation |
| HER2 positive | ~10% | ER- PR- (?) HER2+ | poorly differentiated | poor |
The above is not applied clinically. A panel of immunostains (ER, PR, HER2, EGFR, CK5/6) can reproduce the molecular groupings.[9]
Immunostains for typing and diagnosis
DCIS versus LCIS
Tabular comparison for DCIS versus LCIS:[10][11]
| Disease | E-cadherin | Beta-catenin | 34betaE12 | CAM5.2 (CK8) |
|---|---|---|---|---|
| DCIS | +ve | +ve | -ve | +ve peripheral cytoplasm |
| LCIS | -ve | -ve | +ve perinuclear | +ve perinuclear |
Invasive versus non-invasive
Myoepithelial markers - typically lost in invasive carcinoma:[12]
| Stain | Location | Notes |
|---|---|---|
| p63 | nuclear | up to 10% of invasive tumours +ve[13] |
| Smooth muscle actin (SMA) | cytoplasmic | stains myofibroblasts & blood vessels |
| Calponin | cytoplasmic | stains myofibroblasts & blood vessels |
| Smooth muscle myosin heavy chain (SMM-HC) |
cytoplasmic | stains myofibroblasts & blood vessels |
Usual ductal hyperplasia versus ductal carcinoma in situ
Markers for UDH versus DCIS:[13]
| Disease | CK5/6 | ER |
|---|---|---|
| UDH | diffuse +ve | patchy +ve |
| DCIS | -ve | diffuse +ve |
Lymphovascular invasion
- D2-40 - marks the lymphatic spaces.[14][15]
- CD31 - marks lymphovascular spaces.
- CD34 - marks lymphovascular spaces, less specific than CD31.
- Immunostaining of any sentinel lymph nodes - to look for isolated tumour cells and small lymph node mets.
- Sunnybrook uses CAM5.2.
- ER (estrogen receptor).
- Positive in most breast cancers; +ve in ~75-80%.[16]
- PR (progesterone receptor).
- Positive in most breast cancers; +ve in ~65-70%.[16]
- HER2/neu (HER2).
ER & PR scoring
Nuclear staining:[16]
- Give a percentage, i.e. 0-100%.
- Important cut points: 1% and 10%.
- 0% = negative - not treated.
- <10% = low positivity - treated.
- Important cut points: 1% and 10%.
Notes:
- Normal breast epithelial cells have a patchy staining for ER and PR.
- Evaluated on the invasive component.
HER2 scoring
Immunohistochemical based testing:[16][19][20]
| Score | Staining intensity | Cells stained (%) | Membrane staining | Management | Percentage of cases |
|---|---|---|---|---|---|
| 0 | nil | <=10% | incomplete | No HER2 blocker | ~60% |
| 1+ | minimal-to-weak | >10% | incomplete | No HER2 blocker | ~10% |
| 2+ | weak-to-moderate or intense | >10% and <=30% | complete | Needs SISH or FISH | ~10% |
| 3+ | intense & uniform staining (used to be strong) | >30% (used to >10%) | complete | HER2 blocker | ~20% |
Note for IHC:
- Normal breast epithelial cells do not stain with HER2.
- Evaluated on the invasive component.
- SISH = silver in situ hybridization.
- FISH = fluorescence in situ hybridization.
ISH based testing:[19]
| Result | Ratio criteria | Gene copy number criteria |
|---|---|---|
| Positive | >2.2 HER2/CEP17 | >6.0 copies of HER2/cell |
| Equivocal | 1.8-2.2 HER2/CEP17 | 4.0-6.0 copies of HER2/cell |
| Negative | <1.8 HER2/CEP17 | <4.0 copies of HER2/cell |
Note for ISH:
- Can be called positive based on either ratio criteria or gene copy number criteria.
Clinical
- ER & PR status determine whether a patient will get tamoxifen or other estrogen receptor modulators, such as raloxifene (Evista).
- HER2 status determines whether patient will get traztuzumab (Herceptin) or other HER2/neu modulators.
Characteristics of the subtypes
Invasive ductal carcinoma of the breast
General
- Most common type of invasive breast cancer.
Microscopic
Features:
- Atypical cells:
- Usually >2x RBC diameter.
- Nucleoli common.
- Forming ducts or sheets.
- +/-Mitoses.
- +/-Necrosis.
- Evidence of invasion:
- Atypical nucleus adjacent to adipocyte - diagnostic.
- "Infiltrative" pattern:
- Small glands of variable size within desmoplastic stroma.
- Glands lined by a single layer of cells.
DDx:
IHC
Myoepithelial markers - diagnostic for invasion:
- SMMS -ve.
- p63 -ve.
Prognostic markers - may be useful for metastates:
- ER +ve (diffuse).
- PR +ve (diffuse).
- HER2 -ve.
Invasive lobular carcinoma
- Abbreviated ILC.
- AKA lobular carcinoma.
General
Microscopic
Features:
- "Single file" - cell line-up in a row.
- Cell should not be cohesive -- lymphoma should briefly come to mind.
- primary lymphoma of the breast exists... but it is extremely rare.
- Cell should not be cohesive -- lymphoma should briefly come to mind.
- NO gland formation.
- If it forms glands... it is more likely NST.
- May have signet ring morphology.
- NO desmoplastic reaction, i.e. the stroma surrounding the tumour cells should look benign and undisturbed.
Note:
- Commonly have low grade nuclear features.
Images
More WC images:
Subclassification
- Classic lobular carcinoma.
- Low nuclear grade - NO significant variation of nucleus size.
- Pleomorphic lobular carcinoma.
- Significant nuclear atypia.
Note:
- Some pathologist grade lobular carcinoma like other types and avoid the term "pleomorphic lobular carcinoma."[22]
Medullary breast carcinoma
- AKA medullary carcinoma of the breast.
General
- Uncommon breast cancer subtype.
- Some pathologists don't believe this exists.
Epidemiology:
- Thought to have a better prognosis that no special type (NST).
- May be associated with a BRCA1 mutation.
Gross
- Well-circumscribed border.[23]
Aside - malignant well-circumscribed breast masses - radiologic DDx:[23]
- Medullary breast carcinoma.
- Mucinous breast carcinoma.
- Malignant phyllodes tumour.
- Invasive papillary carcinoma of the breast.
Microscopic
Features:
- Lesion has well-circumscribed border.
- Syncytial growth pattern = clumps of cells with poorly defined cell borders.
- Lymphocytic infiltrate.
- High nuclear grade (as per Nottingham grading system).
- No tubule formation.
IHC
Features - typical:[24]
- ER -ve.
- PR -ve.
- HER2 -ve.
Tubular carcinoma of the breast
- AKA tubular carcinoma.
General
Epidemiology:
- Typically excellent prognosis.
- Hormone receptors commonly present (ER +ve, PR +ve).
- Usually HER2 -ve.
- Classically seen in post-menopausal women.
Note:
- May be seen in association with lobular carcinoma in situ and columnar cell lesions - known as Rosen triad.[25]
- Memory device TLC = Tubular ca., LCIS, Columnar cell lesions.
Microscopic
- Well-formed tubules.
- Typically have angled ducts - "prows" - important feature (low power).
- Myoepithelial cells absent - diagnostic - may be have to appreciated without IHC.
- >70% of the tumour cells should be adjacent to lumen.[29]
- +/- Cribriform spaces.
- Apocrine snouts typical.
- +/-Calcification.
Notes:
- Prow = front of a ship.
- Looks benign to the uninitiated -- important.
DDx:
Image:
IHC
- ER +ve.
- PR +ve.
- HER2 -ve.
- HER2 positivity should prompt consideration of another diagnosis!
Metaplastic breast carcinoma
- AKA metaplastic carcinoma.
General
- May be difficult to diagnose.
- Prognosis - poor.
- Top of the differential diagnosis for spindle cell lesions of the breast.
Microscopic
Features - one of the following:[30][31]
- Malignant mesenchymal elements - either:
- Spindle cells.
- Osseous, chondroid or rhabdoid differentiation.
- Squamous component.
- Non-skin squamous cell carcinoma of the breast = metaplastic breast carcinoma.
Notes:
- Calcifications are uncommon.
- Cytology may be very bland, i.e. it may look very benign.
- May have minimal mitotic activity.
DDx:
- Fibromatosis.
- Malignant phyllodes tumour.
- Primary mammary sarcoma.
- Mammary myofibroblastoma.
- Nodular fasciitis.
- Squamous cell carcinoma of the skin.
Images:
- Metaplastic carcinoma (breastpathology.info).[30]
- Metaplastic carcinoma - case 1 - several images (upmc.edu).
- Metaplastic carcinoma - case 2 - several images (upmc.edu).
Subclassification
- There are various way to subclassify this subtype of breast cancer. This a consequence of physician automomy.
- There is a series of articles on the topic by Wargotz & Norris that is quite old... yet it is mentioned in Sterberg:[32]
- Matrix-producing carcinoma:[33]
- Features: cartilaginous and/or osseous stromal matrix; no osteoclastic giant cells.
- Spindle cell carcinoma:[34]
- Features: (non-malignant) spindle cells.
- Prognosis: better prognosis than other metaplastic carcinomas.
- Carcinosarcoma:[35]
- Features: malignant mesenchymal element.
- Prognosis: survival worse when compared to other metaplastic carcinomas.
- Squamous cell carcinoma of ductal origin:[36]
- Features: purely squamous; metastases are squamous cell carcinoma.
- Metaplastic carcinoma with osteoclastic giant cells:[37]
- Features: osteoclastic giant cells.
- The WHO subclassifies as follows:[38]
- Epithelial - includes: squamous cell carcinoma, adenocarcinoma with spindle cell differentiation, adenosquamous carcinoma.
- Mixed epithelial and mesenchymal - includes: carcinosarcoma, carcinoma with metaplasia (chondroid, osseous).
IHC
- S100 -ve (r/o melanoma).
- AE1/AE3 +ve (epithelial elements only).
- CK7 +ve (epithelial elements only).
- p63 +ve (epithelial elements only).
- Vimentin +ve.
- Desmin -ve.
- EMA -ve. (???)
Invasive micropapillary carcinoma of the breast
- AKA micropapillary carcinoma.
General
- Poor prognosis.
- LVI common.[39]
Microscopic
Features:
- Clear spaces/clefting around (small) nests of tumour - diffuse/through-out the tumour - key feature.
- Described as "small clusters of tumour lying within dilated vascular channel-like spaces".[40]
Note:
- Ductal carcinoma commonly has clefting... but it isn't diffuse.
Images:
- Invasive micropapillary carcinoma (flickr.com/euthman).
- Invasive micropapillary carcinoma - crappy image (breast-cancer.ca).[41]
IHC
- EMA +ve (periphery of nests); described as inside-out pattern.[40]
- E-cadherin +ve (centre of nests). (???)
- p63 +ve/-ve.
Apocrine carcinoma of the breast
General
- Need >=90% apocrine morphology.[42]
Microscopic
Features:[42]
- Prominent nucleoli.
- Often multiple.[43]
- Abundant granular eosinophilic cytoplasm.
- Architecture like invasive ductal carcinomas no special type.
Images:
IHC
Smaller tumours classically:[44]
- AR +ve.
- GCDFP-15 +ve.
Usually:[42]
- ER -ve.
- PR -ve.
Mucinous breast carcinoma
General
- Rare.
- Good prognosis.[45]
- Usually older women.
Gross
- Pale, glistening, jelly-like appearance.
- Well-circumscribed.
Image:
Microscopic
Features:
- Malignant mucin producing glands.
- Mucinous component must comprise >90% of the tumour - required for diagnosis.[46]
- Cells should float in the mucin - key feature.
- Glands typically have irregular edges.
- +/-Vessels within the mucin pools.
DDx:
- DCIS with a mucinous component.
- Mucin has a homogenous appearance, mucin lacks vascularization, mucin pools have a regular border.
Note:
- The amount of mucinous component to call mucinous carcinoma varies by anatomical site.
- All mucinous lesions should be excised.[47]
IHC
- ER +ve.
- PR +ve.
- HER2 -ve.
Adenoid cystic carcinoma of the breast
- AKA breast adenoid cystic carcinoma.
General
- Like tumour of the salivary gland.
- Very rare <0.1% of breast malignancies.[48]
- Good prognosis.[48]
Microscopic
- See: Adenoid cystic carcinoma article.
DDx:
- Cribriform DCIS.
- Collagenous spherulosis.
Images:
Invasive papillary carcinoma of the breast
- AKA intracystic papillary carcinoma of the breast, abbreviated IPC.
- AKA encapsulated papillary carcinoma of the breast, abbreviated EPC.
General
- Very good prognosis[49] - it is similar to DCIS.
- Classical menopausal women.
- ~30% present with bloody discharge.[50]
Microscopic
Features:
- Lesion confined to a duct (intraductal) or cyst (intracystic).
- May have a thick fibrous capsule = encapsulated papillary carcinoma.[50]
- Loss of myoepithelial cells - key feature.
- Neoplastic epithelial cells:
- Nuclear atypia - including: nucleoli, nuclear pleomorphism.
- Abnormal architecture - including cribriform, solid, micropapillary, papillary.
DDx:
IHC
- Loss of myoepithelial markers within the lesion.
Glycogen-rich clear cell carcinoma of the breast
- Abbreviated GRCC.
General
- Very rare.
- Possibly a variant of apocrine carcinoma.[51]
- Prognosis usu. poor.
Microscopic
Features:
- Groups of cells with abundant clear cytoplasm - need to comprise 90% of the tumour.[52]
- Various architectural arrangements: cords, trabeculae, clusters, cribriform.
- Minimal nuclear pleomorphism.
Notes:
- Histologic appearance may be similar to hyalinizing clear cell carcinoma.
DDx:
- Signet-ring carcinoma.
- Lipid-rich carcinoma.
- Apocrine carcinoma.
- Secretory carcinoma.
Image:
Stains
Features:[52]
- PAS +ve.
- PASD -ve.
Others:[52]
- Oil red O -ve.
- Lipid-rich carcinoma +ve.
IHC
Features:[52]
- BRST2 -ve.
- Apocrine carcinoma +ve.
Secretory carcinoma of the breast
- AKA secretory breast carcinoma, abbreviated SBC.
General
- Favourable prognosis.[53]
- Children and adults.
Microscopic
Features:[54]
- Abundant cytoplasm with fine vacuolation - clear/pale or amphophilic.
- Mitoses - uncommon.
- Nucleoli - rare.
- Architecture: solid, papillary, cribriform or microcystic.
DDx:
- Lactational change[55] - glandular architecture.
Images:
- Secretory breast carcinoma - low mag. (webpathology.com).
- Secretory breast carcinoma - high mag. (webpathology.com).
- Secretory breast carcinoma (nature.com).
IHC
Triple negative (ER -ve, PR -ve, HER2 -ve).[53]
Others:
- Alpha-lactalbumin +ve.
- S-100 protein +ve.
- Polyclonal CEA +ve.
Molecular pathology
Characteristic translocation:[53]
- t(12;15).
- ETV6-NTRK3.
Grading breast cancer
Most common system: Nottingham (aka Scarff-Bloom-Richardson) which is based on:
- Nuclear grade.
- Small, regular (1.5-2x RBC dia.) = 1.
- Moderated variability = 2.
- Marked variation (>2.5x RBC dia.) = 3.
- Tubule formation.
- Majority of tumour - tubules >75% = 1.
- Moderate - 10% to 75% = 2.
- Minimal <10% = 3.
- Mitotic rate.
- 0-5 mitosis/10 HPF (1.52 mm^2 --or-- 0.0152 mm^2 * 10) = 1.
- 6-10 mitosis/10 HPF (1.52 mm^2) = 2.
- >11 mitosis/10 HPF (1.52 mm^2) = 3.
Mnemonic: TMN = tubule formation, mitotic rate, nuclear grade.
Notes:
- Elston & Ellis devised the system that is used.[56] They also wrote a follow-up article in 2002.[57]
Note about mitosis counting
- One MUST adjust for the size of the field of view.
- Most of the Resident scopes have an eye piece diameter of 22 mm. Therefore, the field diameter at 40 X is approximately 22 mm / 40 X ~= 0.55 mm and the field of view is pi/4*(0.55 mm)^2 = 0.2376 mm^2.
- Thus, on a resident scope (with a FOV of 0.2376 mm^2) one should sample 6 or 7 fields of view (FsOV).
- Calculation: 1.52 mm^2 (sampling area) / 0.2376 mm^2 (area / FOV ) = 6.40 FsOV.
- Thus, on a resident scope (with a FOV of 0.2376 mm^2) one should sample 6 or 7 fields of view (FsOV).
- RANT: Sampling 10 fields, where the field of view (FOV) is 0.152 mm^2, is not the same as sampling ten fields, where the FOV is 0.312 mm^2. It surprises me that Elston & Ellis ignore the fact that "10 HPFs" on different microscopes represent different sample areas and that they do not standardize the sampling area.
Calculating Nottingham score
- Grade I = 3-5 points.
- Grade II = 6-7 points.
- Grade III = 8-9 points.
Notes:
- I've found most tumours are grade II.
- The mitotic score is usually 1/3.
- The nuclear score is rarely 1/3 -- even in the tubular subtype.[58]
Staging breast cancer
Sentinel lymph node sampling in breast cancer
General
- Selective sampling of lymph nodes.
- Used for staging.
- Positive LNs = poorer prognosis.
Notes:
- If there is no palpable disease, there is no mortality benefit from axillary lymph node dissection, i.e. positive axillary lymph nodes can be left in situ without affecting outcome.[59]
- This does not negate the fact that a positive sentinel LN biopsy (vs. negative sentinel LN biopsy) portends a poorer prognosis.
Microscopic
Features:
- Atypical cells.
- Nuclear changes of malignancy:
- Nuclear enlargement + variation in size.
- Variation in shape.
- Hyperchromasia and variation in staining.
- Usually in the subcapsular sinuses.
- Nuclear changes of malignancy:
Pitfalls:
- Naevus cell rests.[60]
IHC
Some hospitals use:
- CAM5.2 (LMWK) - to look for isolated tumour cells and small lymph node metstases.
N stage
Sampling usually selective, i.e. sentinel lymph nodes only.
Indictionas for lymph node sampling
Indications for lymph node sampling:[61]
- Extensive DCIS.
- Biopsy suspicious for invasion or with microinvasion.
- Clinical findings (large palable mass) or radiology findings (irregular features) suggestive of invasion.
- Planned mastectomy.
Definitions
Definitions:[62]
- Isolated tumour cells: <=0.2 mm or <=200 cells -- in a single cross-section. †
- Micrometastasis: <=0.2 cm and ( >0.2 mm or >200 cells ).
- Macrometastasis: >0.2 cm.
Notes:
- † The American Cancer Society web site says "or".[62] The CAP protocol says "and/or" and notes it is all subjective.
- Isolated tumour cells are essentially ignored if the there is at least one macrometastasis.
Details
Lymph nodes:[63]
- pN0: nil.
- pN0(i+): <=0.2 mm and <200 cells.
- pN1: 1-3 axillary LNs or internal mammary LNs.
- pN1mi: <=0.2 cm and ( >0.2 mm or >=200 cells ).
- pN1a.
- pN1b.
- PN1c.
- pN2 4-9 positive LNs; internal mammary LNs or axillary LNs.
- pN3.
T stage
- pT1: <= 20 mm.
- pT1mic <= 1 mm.
- pT1a > 1 mm and <= 5 mm.
- pT1b > 5 mm and <= 10 mm.
- pT1c > 10 mm and <= 20 mm.
- pT2: > 20 mm and <= 50 mm
- pT3: > 50 mm.
- pT4: chest wall or skin involvement.
Notes:
- Values should be rounded to the nearest millimetre.
- Therefore:
- 1.4 mm would be pT1mic.
- 1.5 mm would be pT1a.
- Therefore:
M stage
Distant metastasis:
- cM0(i+) <=0.2 mm focus of tumour cells, without clinical signs and symptoms.
- pM1 focus of tumour cells > 0.2 mm.
Lymphovascular invasion
In the context of breast pathology, the Rosen criteria for LVI are widely excepted, and are as follows:[66][67]
- Must be outside of the tumour proper.
- LVI is usually very close -- typically within 0.1 cm.
- Contour of cells should differ from possible vessel wall.
- DCIS with retraction artifact mimicing LVI has a contour that matches its surrounding fibrous tissue.
- Endothelium (usu. flat) should be visible.
- Lymphatics are found adjacent to blood vessels - vessels should be present in the vicinity.
Memory device LUBE-O:
- LVI has a Unique contour, Blood vessels and Endothelium in the vicinity, and is Outside of the tumour.
Note:
- LVI does not affect the stage.
Other
Paget's disease
General
- Associated with underlying breast carcinoma.[68]
Notes:
- Unrelated to Paget disease of the bone.
Microscopic
Features:[68]
- Cells in the epidermis:
- Epitheliod morphology (round/ovoid).
- Cells nested or single.
- Clear/pale cytoplasm key feature - may also be eosinophilic.
- Large nucleoli.
Images:
IHC & DDx:
- See Paget disease.
Trivia
Tumour size and lymph node metastases
There is a paper[69] that calculates the probability of lymph node mets based on tumour size. The developed formula is:
Where:
- = the probability of the lymph nodes being positive.
- D = the largest dimension of the tumour in millimetres.
- Z = 1.0041.
- = 0.019.
Selected values
| Tumour size (mm) | Probability |
| 5 | 9 % |
| 10 | 17 % |
| 15 | 25 % |
| 20 | 32 % |
| 25 | 38 % |
| 30 | 44 % |
| 35 | 49 % |
| 40 | 54 % |
| 45 | 58 % |
| 50 | 62 % |
Natural history
There is a theory that up to 22% of small (radiographically detected) breast tumours regress, based on an analysis in a large population.[70] The study is supported by NCI's SEER data.[71] Also, it generated many comments.[70]
Missed macrometastases
The effect of missed macrometastases is small; this implies using IHC to look for isolated tumour cells is money that isn't well spent.[72]
See also
References
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1143. ISBN 0-7216-0187-1.
- ↑ URL: http://www.breastpathology.info/Case_of_the_month/2007/COTM_1007%20discussion.html. Accessed on: 28 November 2010.
- ↑ URL: http://emedicine.medscape.com/article/1947145-overview. Accessed on: 24 August 2012.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 113705
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600185
- ↑ 6.0 6.1 Online 'Mendelian Inheritance in Man' (OMIM) 192090
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1078. ISBN 978-1416031215.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 547. ISBN 978-1416054542.
- ↑ Tang, P.; Skinner, KA.; Hicks, DG. (Sep 2009). "Molecular classification of breast carcinomas by immunohistochemical analysis: are we ready?". Diagn Mol Pathol 18 (3): 125-32. doi:10.1097/PDM.0b013e31818d107b. PMID 19704256.
- ↑ O'Malley, Frances P.; Pinder, Sarah E. (2006). Breast Pathology: A Volume in Foundations in Diagnostic Pathology series (1st ed.). Churchill Livingstone. pp. 275. ISBN 978-0443066801.
- ↑ Yeh IT, Mies C (March 2008). "Application of immunohistochemistry to breast lesions". Arch. Pathol. Lab. Med. 132 (3): 349-58. PMID 18318578. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=132&page=349.
- ↑ Lester, Susan Carole (2010). Manual of Surgical Pathology (3rd ed.). Saunders. pp. 88. ISBN 978-0-323-06516-0.
- ↑ 13.0 13.1 O'Malley, Frances P.; Pinder, Sarah E. (2006). Breast Pathology: A Volume in Foundations in Diagnostic Pathology series (1st ed.). Churchill Livingstone. pp. 276. ISBN 978-0443066801.
- ↑ Ordóñez NG (March 2006). "Podoplanin: a novel diagnostic immunohistochemical marker". Adv Anat Pathol 13 (2): 83-8. doi:10.1097/01.pap.0000213007.48479.94. PMID 16670463.
- ↑ Kahn HJ, Marks A (September 2002). "A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors". Lab. Invest. 82 (9): 1255-7. PMID 12218087.
- ↑ 16.0 16.1 16.2 16.3 16.4 Lester, Susan Carole (2005). Manual of Surgical Pathology (2nd ed.). Saunders. pp. 241-2. ISBN 978-0443066450.
- ↑ Gallardo, A.; Lerma, E.; Escuin, D.; Tibau, A.; Muñoz, J.; Ojeda, B.; Barnadas, A.; Adrover, E. et al. (Apr 2012). "Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas.". Br J Cancer 106 (8): 1367-73. doi:10.1038/bjc.2012.85. PMID 22454081.
- ↑ Jensen, JD.; Knoop, A.; Laenkholm, AV.; Grauslund, M.; Jensen, MB.; Santoni-Rugiu, E.; Andersson, M.; Ewertz, M. (Dec 2011). "PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome in HER2-positive early-stage breast cancer patients treated with adjuvant chemotherapy and trastuzumab.". Ann Oncol. doi:10.1093/annonc/mdr546. PMID 22172323.
- ↑ 19.0 19.1 Lester, Susan Carole (2010). Manual of Surgical Pathology (3rd ed.). Saunders. pp. 87. ISBN 978-0-323-06516-0.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2009/InvasiveBreast_09protocol.pdf. Accessed on: 27 November 2011.
- ↑ URL: http://www.asco.org/ascov2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=33006. Accessed on: 19 April 2011.
- ↑ MUA. Jan 22, 2009.
- ↑ 23.0 23.1 Yoo, JL.; Woo, OH.; Kim, YK.; Cho, KR.; Yong, HS.; Seo, BK.; Kim, A.; Kang, EY. (Oct 2010). "Can MR Imaging contribute in characterizing well-circumscribed breast carcinomas?". Radiographics 30 (6): 1689-702. doi:10.1148/rg.306105511. PMID 21071383.
- ↑ Matkovic, B.; Juretic, A.; Separovic, V.; Novosel, I.; Separovic, R.; Gamulin, M.; Kruslin, B.. "Immunohistochemical analysis of ER, PR, HER-2, CK 5/6, p63 and EGFR antigen expression in medullary breast cancer.". Tumori 94 (6): 838-44. PMID 19267102.
- ↑ Brandt, SM.; Young, GQ.; Hoda, SA. (May 2008). "The "Rosen Triad": tubular carcinoma, lobular carcinoma in situ, and columnar cell lesions.". Adv Anat Pathol 15 (3): 140-6. doi:10.1097/PAP.0b013e31816ff313. PMID 18434766.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1146. ISBN 0-7216-0187-1.
- ↑ URL: http://www.bweems.com/nsj3mp2.jpg.
- ↑ URL: http://surgpathcriteria.stanford.edu/breast/tubularcabr/.
- ↑ Stalsberg, H.; Hartmann, WH. (May 2000). "The delimitation of tubular carcinoma of the breast.". Hum Pathol 31 (5): 601-7. PMID 10836300.
- ↑ 30.0 30.1 URL: http://www.breastpathology.info/Case_of_the_month/2007/COTM_0807%20discussion.html. Accessed on: 28 November 2010.
- ↑ Barnes, PJ.; Boutilier, R.; Chiasson, D.; Rayson, D. (May 2005). "Metaplastic breast carcinoma: clinical-pathologic characteristics and HER2/neu expression.". Breast Cancer Res Treat 91 (2): 173-8. doi:10.1007/s10549-004-7260-y. PMID 15868445.
- ↑ Mills, Stacey E; Carter, Darryl; Greenson, Joel K; Reuter, Victor E; Stoler, Mark H (2009). Sternberg's Diagnostic Surgical Pathology (5th ed.). Lippincott Williams & Wilkins. pp. 328. ISBN 978-0781779425.
- ↑ Wargotz, ES.; Norris, HJ. (Jul 1989). "Metaplastic carcinomas of the breast. I. Matrix-producing carcinoma.". Hum Pathol 20 (7): 628-35. PMID 2544506.
- ↑ Wargotz, ES.; Deos, PH.; Norris, HJ. (Aug 1989). "Metaplastic carcinomas of the breast. II. Spindle cell carcinoma.". Hum Pathol 20 (8): 732-40. PMID 2473024.
- ↑ Wargotz, ES.; Norris, HJ. (Oct 1989). "Metaplastic carcinomas of the breast. III. Carcinosarcoma.". Cancer 64 (7): 1490-9. PMID 2776108.
- ↑ Wargotz, ES.; Norris, HJ. (Jan 1990). "Metaplastic carcinomas of the breast. IV. Squamous cell carcinoma of ductal origin.". Cancer 65 (2): 272-6. PMID 2153044.
- ↑ Wargotz, ES.; Norris, HJ. (Nov 1990). "Metaplastic carcinomas of the breast: V. Metaplastic carcinoma with osteoclastic giant cells.". Hum Pathol 21 (11): 1142-50. PMID 2227922.
- ↑ O'Malley, Frances P.; Pinder, Sarah E. (2006). Breast Pathology: A Volume in Foundations in Diagnostic Pathology series (1st ed.). Churchill Livingstone. pp. 214. ISBN 978-0443066801.
- ↑ Yu, JI.; Choi, DH.; Park, W.; Huh, SJ.; Cho, EY.; Lim, YH.; Ahn, JS.; Yang, JH. et al. (Jun 2010). "Differences in prognostic factors and patterns of failure between invasive micropapillary carcinoma and invasive ductal carcinoma of the breast: matched case-control study.". Breast 19 (3): 231-7. doi:10.1016/j.breast.2010.01.020. PMID 20304650.
- ↑ 40.0 40.1 Yamaguchi, R.; Tanaka, M.; Kondo, K.; Yokoyama, T.; Kaneko, Y.; Yamaguchi, M.; Ogata, Y.; Nakashima, O. et al. (Aug 2010). "Characteristic morphology of invasive micropapillary carcinoma of the breast: an immunohistochemical analysis.". Jpn J Clin Oncol 40 (8): 781-7. doi:10.1093/jjco/hyq056. PMID 20444748.
- ↑ URL: http://www.breast-cancer.ca/type/micropapillary-breast-carcinoma.htm. Accessed on: 30 May 2012.
- ↑ 42.0 42.1 42.2 O'Malley, Frances P.; Pinder, Sarah E. (2006). Breast Pathology: A Volume in Foundations in Diagnostic Pathology series (1st ed.). Churchill Livingstone. pp. 217. ISBN 978-0443066801.
- ↑ O'Malley, FP.; Bane, A. (Jan 2008). "An update on apocrine lesions of the breast.". Histopathology 52 (1): 3-10. doi:10.1111/j.1365-2559.2007.02888.x. PMID 18171412.
- ↑ Honma, N.; Takubo, K.; Akiyama, F.; Sawabe, M.; Arai, T.; Younes, M.; Kasumi, F.; Sakamoto, G. (Aug 2005). "Expression of GCDFP-15 and AR decreases in larger or node-positive apocrine carcinomas of the breast.". Histopathology 47 (2): 195-201. doi:10.1111/j.1365-2559.2005.02181.x. PMID 16045781.
- ↑ Barkley, CR.; Ligibel, JA.; Wong, JS.; Lipsitz, S.; Smith, BL.; Golshan, M. (Oct 2008). "Mucinous breast carcinoma: a large contemporary series.". Am J Surg 196 (4): 549-51. doi:10.1016/j.amjsurg.2008.06.013. PMID 18809061.
- ↑ Dogan, E.; Aksoy, S.; Dizdar, O.; Arslan, C.; Dede, DS.; Ozisik, Y.; Altundag, K.. "Pure mucinous carcinoma of the breast: a single center experience.". J BUON 16 (3): 565-7. PMID 22006768.
- ↑ Jacobs, TW.; Connolly, JL.; Schnitt, SJ. (Sep 2002). "Nonmalignant lesions in breast core needle biopsies: to excise or not to excise?". Am J Surg Pathol 26 (9): 1095-110. PMID 12218567.
- ↑ 48.0 48.1 Boujelbene, N.; Khabir, A.; Boujelbene, N.; Jeanneret Sozzi, W.; Mirimanoff, RO.; Khanfir, K. (Dec 2011). "Clinical review - Breast adenoid cystic carcinoma.". Breast. doi:10.1016/j.breast.2011.11.006. PMID 22154460.
- ↑ Rakha, EA.; Gandhi, N.; Climent, F.; van Deurzen, CH.; Haider, SA.; Dunk, L.; Lee, AH.; Macmillan, D. et al. (Aug 2011). "Encapsulated papillary carcinoma of the breast: an invasive tumor with excellent prognosis.". Am J Surg Pathol 35 (8): 1093-103. doi:10.1097/PAS.0b013e31821b3f65. PMID 21753694.
- ↑ 50.0 50.1 Rodríguez, MC.; Secades, AL.; Angulo, JM. (Nov 2010). "Best cases from the AFIP: intracystic papillary carcinoma of the breast.". Radiographics 30 (7): 2021-7. doi:10.1148/rg.307105003. PMID 21057133.
- ↑ Hayes, MM.; Seidman, JD.; Ashton, MA. (Aug 1995). "Glycogen-rich clear cell carcinoma of the breast. A clinicopathologic study of 21 cases.". Am J Surg Pathol 19 (8): 904-11. PMID 7611537.
- ↑ 52.0 52.1 52.2 52.3 52.4 Thondavadi, SR.; Krishnamurthy, J.; Gubbanna, VM.. "A case report of glycogen-rich clear cell carcinoma of breast.". Indian J Pathol Microbiol 53 (2): 374-5. doi:10.4103/0377-4929.64289. PMID 20551566.
- ↑ 53.0 53.1 53.2 Vasudev, P.; Onuma, K. (Dec 2011). "Secretory breast carcinoma: unique, triple-negative carcinoma with a favorable prognosis and characteristic molecular expression.". Arch Pathol Lab Med 135 (12): 1606-10. doi:10.5858/arpa.2010-0351-RS. PMID 22129193.
- ↑ Laé, M.; Fréneaux, P.; Sastre-Garau, X.; Chouchane, O.; Sigal-Zafrani, B.; Vincent-Salomon, A. (Feb 2009). "Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum.". Mod Pathol 22 (2): 291-8. doi:10.1038/modpathol.2008.184. PMID 19011601.
- ↑ Vesoulis, Z.; Kashkari, S.. "Fine needle aspiration of secretory breast carcinoma resembling lactational changes. A case report.". Acta Cytol 42 (4): 1032-6. PMID 9684599.
- ↑ Elston CW, Ellis IO (September 2002). "Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991; 19; 403-410". Histopathology 41 (3A): 151–2, discussion 152–3. PMID 12405945.
- ↑ Elston CW, Ellis IO (November 1991). "Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up". Histopathology 19 (5): 403–10. PMID 1757079.
- ↑ MUA. 20 January 2009.
- ↑ Giuliano AE, Hunt KK, Ballman KV, et al. (February 2011). "Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial". JAMA 305 (6): 569–75. doi:10.1001/jama.2011.90. PMID 21304082.
- ↑ URL: http://www.breastpathology.info/Case_of_the_month/2007/COTM_1107%20discussion.html. Accessed on: 28 November 2010.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2009/InvasiveBreast_09protocol.pdf. Accessed on: 2 April 2012.
- ↑ 62.0 62.1 URL: http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-staging. Accessed on: 8 July 2010.
- ↑ URL: http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-staging. Accessed on: 8 July 2010.
- ↑ URL: http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-staging. Accessed on: 8 July 2010.
- ↑ URL: http://www.cancerhelp.org.uk/type/breast-cancer/treatment/tnm-breast-cancer-staging. Accessed on: 9 July 2010.
- ↑ Rosen, PP. (1983). "Tumor emboli in intramammary lymphatics in breast carcinoma: pathologic criteria for diagnosis and clinical significance.". Pathol Annu 18 Pt 2: 215-32. PMID 6674861.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2009/InvasiveBreast_09protocol.pdf. Accessed on: 5 August 2011.
- ↑ 68.0 68.1 URL: http://emedicine.medscape.com/article/1101235-diagnosis
- ↑ Porembka, MR.; Abraham, RL.; Sefko, JA.; Deshpande, AD.; Jeffe, DB.; Margenthaler, JA. (Oct 2008). "Factors associated with lymph node assessment in ductal carcinoma in situ: analysis of 1988-2002 seer data.". Ann Surg Oncol 15 (10): 2709-19. doi:10.1245/s10434-008-9947-5. PMID 18483831. http://onlinelibrary.wiley.com/doi/10.1002/cncr.24592/pdf.
- ↑ 70.0 70.1 Zahl, PH.; Maehlen, J.; Welch, HG. (Nov 2008). "The natural history of invasive breast cancers detected by screening mammography.". Arch Intern Med 168 (21): 2311-6. doi:10.1001/archinte.168.21.2311. PMID 19029493.
- ↑ Jatoi, I.; Anderson, WF. (May 2009). "Breast cancer overdiagnosis with screening mammography.". Arch Intern Med 169 (10): 999-1000, author reply 1000-1. doi:10.1001/archinternmed.2009.95. PMID 19468099.
- ↑ Weaver, DL.; Ashikaga, T.; Krag, DN.; Skelly, JM.; Anderson, SJ.; Harlow, SP.; Julian, TB.; Mamounas, EP. et al. (Feb 2011). "Effect of occult metastases on survival in node-negative breast cancer.". N Engl J Med 364 (5): 412-21. doi:10.1056/NEJMoa1008108. PMID 21247310.