Difference between revisions of "Gastrointestinal tract polyps"
| (126 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Image:Polyp-2.jpeg|thumb|right|Endoscopic image of a gastrointestinal polyp.]] | |||
'''Gastrointestinal tract polyps''', also '''gastrointestinal polyps''' or '''GI polyps''', are the bread & butter of a GI pathologists workload. Some of 'em are benign... some pre-malignant... some malignant... some weird. Most GI polyps are from the intestine, i.e. intestinal polyps. | '''Gastrointestinal tract polyps''', also '''gastrointestinal polyps''' or '''GI polyps''', are the bread & butter of a GI pathologists workload. Some of 'em are benign... some pre-malignant... some malignant... some weird. Most GI polyps are from the intestine, i.e. intestinal polyps. | ||
| Line 26: | Line 27: | ||
{{familytree | D | | | | E | | | | F | | G |D=Nuclear changes|E=No nuc. change|F=Serrated|G=Not serrated}} | {{familytree | D | | | | E | | | | F | | G |D=Nuclear changes|E=No nuc. change|F=Serrated|G=Not serrated}} | ||
{{familytree | |!| | | |,|-|^|-|.| | | |!| | | |!| |}} | {{familytree | |!| | | |,|-|^|-|.| | | |!| | | |!| |}} | ||
{{familytree | H | | I | | J | | K | | L |H=Polypoid adenoma<br>(below)|I=Serrated|J=Not serrated|K=[[sessile serrated adenoma|SSA]] | {{familytree | H | | I | | J | | K | | L |H=Polypoid adenoma<br>(below)|I=Serrated|J=Not serrated|K=[[sessile serrated adenoma|SSA]] versus HP|L=Normal versus VA}} | ||
{{familytree | | | | | |!| | | |!| | | | | | | | | |}} | {{familytree | | | | | |!| | | |!| | | | | | | | | |}} | ||
{{familytree | | | | | M | | N | | | | | | | | |M=[[Hyperplastic polyp|HP]]|N=See misc.<br>polyps (below)}} | {{familytree | | | | | M | | N | | | | | | | | |M=[[Hyperplastic polyp|HP]]|N=See misc.<br>polyps (below)}} | ||
| Line 89: | Line 90: | ||
! Image | ! Image | ||
|- | |- | ||
| Normal mucosa / no pathology | | [[Normal colorectal mucosa|Normal mucosa]] / no pathology | ||
| test tubes in a rack-like morphology | | test tubes in a rack-like morphology | ||
| small nuclei, abundant goblet cells | | small nuclei, abundant goblet cells | ||
| common / benign | | common / benign | ||
| moderate inflammation is normal | | moderate inflammation is normal | ||
| [[colonic spirochetes]], [[cryptosporidiosis]], [[microscopic colitis]], CMV colitis | | missed lesion, [[colonic spirochetes]], [[cryptosporidiosis]], [[microscopic colitis]], [[CMV colitis]] | ||
| [ | | [[Image:Rectum - intermed mag.jpg|thumb|center|150px| Normal rectum (WC)]] | ||
|- | |- | ||
| [[Hyperplastic polyp]] | | [[Hyperplastic polyp]] | ||
| Line 103: | Line 104: | ||
| may be syndromic, e.g. [[hyperplastic polyposis syndrome]] | | may be syndromic, e.g. [[hyperplastic polyposis syndrome]] | ||
| [[sessile serrated adenoma]] | | [[sessile serrated adenoma]] | ||
| [ | | [[Image:Hyperplastic polyp -- intermed mag.jpg |thumb|center|150px| HP (WC)]] | ||
|- | |- | ||
| [[Traditional adenoma]] | | [[Traditional adenoma]] | ||
| Line 111: | Line 112: | ||
| [[tubular adenoma of the gastrointestinal tract|tubular adenoma]], [[tubulovillous adenoma]], [[villous adenoma]] | | [[tubular adenoma of the gastrointestinal tract|tubular adenoma]], [[tubulovillous adenoma]], [[villous adenoma]] | ||
| [[traditional serrated adenoma]], reactive changes (inflammation) | | [[traditional serrated adenoma]], reactive changes (inflammation) | ||
| [ | | [[Image:Tubular_adenoma_4_low_mag.jpg|thumb|center|150px| TA (WC)]] | ||
|} | |} | ||
| Line 130: | Line 131: | ||
| AKA sessile serrated polyp | | AKA sessile serrated polyp | ||
| hyperplastic polyp | | hyperplastic polyp | ||
| [ | | [[Image:Sessile_serrated_adenoma_2_low_mag.jpg|thumb|center|150px|SSA (WC)]] | ||
|- | |- | ||
| [[Traditional serrated adenoma]] (TSA) | | [[Traditional serrated adenoma]] (TSA) | ||
| Line 138: | Line 139: | ||
| called "traditional" to differentiate from SSA | | called "traditional" to differentiate from SSA | ||
| traditional serrated adenoma (esp. villous adenoma) | | traditional serrated adenoma (esp. villous adenoma) | ||
| [ | | [[Image:Traditional_serrated_adenoma_low_mag.jpg|thumb|center|150px|TSA (WC)]] | ||
|- | |- | ||
| [[Juvenile polyp]] (retention polyp) | | [[Juvenile polyp]] (retention polyp) | ||
| Line 146: | Line 147: | ||
| may be part of [[juvenile polyposis syndrome]] | | may be part of [[juvenile polyposis syndrome]] | ||
| inflammatory pseudopolyp | | inflammatory pseudopolyp | ||
| [ | | [[Image:Gastric_juvenile_polyp_-_very_low_mag.jpg|thumb|center|150px|Gastric JP (WC)]] | ||
|- | |- | ||
| [[Inflammatory pseudopolyp]] | | [[Inflammatory pseudopolyp]] | ||
| Line 154: | Line 155: | ||
| only seen in [[IBD]]; Dx implies IBD | | only seen in [[IBD]]; Dx implies IBD | ||
| juvenile polyp | | juvenile polyp | ||
| Image | | [[Image:Inflammatory polyp -- low mag.jpg|thumb|center|120px|IP (WC)]] | ||
|- | |- | ||
| [[Peutz-Jeghers polyp]] (PJP) | | [[Peutz-Jeghers polyp]] (PJP) | ||
| Line 162: | Line 163: | ||
| PJP not pre-malignant lesion in itself; see ''[[Peutz-Jeghers syndrome]]'' | | PJP not pre-malignant lesion in itself; see ''[[Peutz-Jeghers syndrome]]'' | ||
| normal, classically in the small bowel | | normal, classically in the small bowel | ||
| [ | | [[Image:Peutz-Jeghers_syndrome_polyp.jpg|thumb|center|120px|PJP (WC)]] | ||
|} | |} | ||
| Line 175: | Line 176: | ||
===Adenomatous vs. hyperplastic=== | ===Adenomatous vs. hyperplastic=== | ||
Adenomatous polyps & hyperplastic polyps - a comparison (adapted from Li and Burgart<ref>{{cite journal |author=Li SC, Burgart L |title=Histopathology of serrated adenoma, its variants, and differentiation from conventional adenomatous and hyperplastic polyps |journal=Arch. Pathol. Lab. Med. |volume=131 |issue=3 |pages=440-5 |year=2007 |month=March |pmid=17516746 |doi= |url=http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=131&page=440}}</ref>): | Adenomatous polyps & hyperplastic polyps - a comparison (adapted from Li and Burgart<ref name=pmid17516746>{{cite journal |author=Li SC, Burgart L |title=Histopathology of serrated adenoma, its variants, and differentiation from conventional adenomatous and hyperplastic polyps |journal=Arch. Pathol. Lab. Med. |volume=131 |issue=3 |pages=440-5 |year=2007 |month=March |pmid=17516746 |doi= |url=http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=131&page=440}}</ref>): | ||
{| class="wikitable" | {| class="wikitable" | ||
! Attribute | |||
! Hyperplastic polyp (HP) | |||
! Sessile serrated adenoma (SSA) | |||
! Traditional serrated adenoma (TSA) | |||
! Traditional adenoma'''<br>-tubular adenoma<br>-tubulovillous adenoma<br>-villous adenoma | |||
|- | |- | ||
|Classic location ||rectum/left colon ||right colon ||rectum/left colon ||rectum/left colon | |Classic location ||rectum/left colon ||right colon ||rectum/left colon ||rectum/left colon | ||
| Line 198: | Line 203: | ||
|- | |- | ||
|Image(s) | |Image(s) | ||
| [ | | [[Image:Hyperplastic polyp -- intermed mag.jpg |thumb|center|150px|HP (WC)]] | ||
| [ | | [[Image:Sessile_serrated_adenoma_2_low_mag.jpg|thumb|center|150px|SSA (WC)]] | ||
| [ | | [[Image:Traditional_serrated_adenoma_low_mag.jpg|thumb|center|150px|TSA (WC)]] | ||
|[ | |[[Image:Tubular_adenoma_2_low_mag.jpg|thumb|center|150px|TA (WC)]] | ||
|} | |} | ||
Normal colonic mucosa: | Normal colonic mucosa: | ||
| Line 207: | Line 212: | ||
*Abundant goblet cells. | *Abundant goblet cells. | ||
*Moderate inflammation. | *Moderate inflammation. | ||
*Paneth | *[[Paneth cell]]s - present in right colon. | ||
*Glands - straight, no branching; "test tube" shape. | *Glands - straight, no branching; "test tube" shape. | ||
| Line 213: | Line 218: | ||
=Normal= | =Normal= | ||
==Normal colorectal mucosa== | |||
===General=== | ===General=== | ||
*Endoscopists go after anything that is polypoid... and that may be normal. | *Endoscopists go after anything that is polypoid... and that may be normal. | ||
===Microscopic=== | ===Microscopic=== | ||
Features: | Features: | ||
| Line 222: | Line 227: | ||
*Minimal palisading. | *Minimal palisading. | ||
**Nuclei <3:1 = height:width. | **Nuclei <3:1 = height:width. | ||
*No nuclear pseudostratification. | *No nuclear pseudostratification. † | ||
*Deep part of crypt more hyperchromatic than superficial component. | *Deep part of crypt is more hyperchromatic than superficial component - '''important'''. | ||
**The surface should be lighter staining than the deeper aspect, i.e. the deeper glands are dark blue and the superficial gland are light blue. | |||
Images: | Note: | ||
* † May be seen in [[reactive changes]]. | |||
DDx (colorectal mucosa with minimal changes): | |||
*[[CMV colitis]]. | |||
*[[Cryptosporidiosis]]. | |||
*[[Intestinal spirochetosis]]. | |||
*[[Lymphocytic colitis]]. | |||
*[[Collagenous colitis]]. | |||
====Images==== | |||
<gallery> | |||
Image:Rectum - low mag.jpg | Rectum - low mag. (WC) | |||
Image:Rectum - intermed mag.jpg | Rectum - intermed. mag. (WC) | |||
Image:Rectum - alt - intermed mag.jpg | Rectum - intermed. mag. (WC) | |||
Image:Rectum - high mag.jpg | Rectum - high mag. (WC) | |||
</gallery> | |||
www: | |||
*[http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/images/col10he.jpg Normal colorectal mucosa (uwa.edu.au)].<ref>URL: [http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/git.htm http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/git.htm]. Accessed on: 18 October 2012.</ref> | *[http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/images/col10he.jpg Normal colorectal mucosa (uwa.edu.au)].<ref>URL: [http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/git.htm http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/git.htm]. Accessed on: 18 October 2012.</ref> | ||
*[http://www.siumed.edu/~dking2/erg/GI027b.htm Colon (siumed.edu)]. | *[http://www.siumed.edu/~dking2/erg/GI027b.htm Colon (siumed.edu)]. | ||
*[http://www.gwc.maricopa.edu/class/bio202/Digestive/DigestHisto/ColonA.htm Normal colorectal mucosa (maricopa.edu)]. | *[http://www.gwc.maricopa.edu/class/bio202/Digestive/DigestHisto/ColonA.htm Normal colorectal mucosa (maricopa.edu)]. | ||
| Line 234: | Line 256: | ||
====Normal==== | ====Normal==== | ||
<pre> | <pre> | ||
Cecum, Biopsy: | |||
- | - Colorectal-type mucosa within normal limits. | ||
</pre> | </pre> | ||
<pre> | <pre> | ||
Right Colon, Biopsy: | |||
- | - Colonic mucosa within normal limits. | ||
</pre> | |||
<pre> | |||
Transverse Colon, Biopsy: | |||
- Colonic mucosa within normal limits. | |||
</pre> | |||
<pre> | |||
Left Colon, Biopsy: | |||
- Colonic mucosa within normal limits. | |||
</pre> | |||
<pre> | |||
Rectum, Biopsy: | |||
- Colorectal mucosa within normal limits. | |||
</pre> | </pre> | ||
= | =====Block letters===== | ||
<pre> | |||
=== | SIGMOID COLON, BIOPSY: | ||
- COLORECTAL-TYPE MUCOSA WITHIN NORMAL LIMITS. | |||
</pre> | |||
<pre> | |||
COLON, 70 CM, BIOPSY: | |||
- COLORECTAL-TYPE MUCOSA WITHIN NORMAL LIMITS. | |||
</pre> | |||
=====Polypoid fragments===== | |||
<pre> | |||
POLYP, SIGMOID COLON, BIOPSY: | |||
- POLYPOID FRAGMENT OF COLORECTAL-TYPE MUCOSA WITHIN NORMAL LIMITS. | |||
</pre> | |||
=====Mucosa and submucosa===== | |||
<pre> | |||
POLYP, SIGMOID COLON, BIOPSY: | |||
- COLONIC MUCOSA AND SUBMUCOSA WITHIN NORMAL LIMITS. | |||
</pre> | |||
====Lymphoid nodule present==== | |||
* | *Lymphoid nodules manifest endoscopically as a small polypoid protuberances. It is worthwhile to report the presence of lymphoid nodules as they reassure the endoscopist that they probably sampled the abnormality they saw. | ||
<pre> | |||
POLYP, RECTUM, BIOPSY: | |||
- RECTAL MUCOSA WITHIN NORMAL LIMITS WITH A MORPHOLOGICALLY BENIGN LYMPHOID AGGREGATE. | |||
</pre> | |||
<pre> | <pre> | ||
COLON, RIGHT SIDE, BIOPSY: | |||
- | - COLONIC MUCOSA WITH MORPHOLOGICALLY BENIGN LYMPHOID AGGREGATES, | ||
NO SIGNIFICANT PATHOLOGY. | |||
</pre> | </pre> | ||
=====Submucosa present===== | |||
<pre> | <pre> | ||
POLYP, ASCENDING COLON, BIOPSY: | |||
- | - COLONIC MUCOSA AND SUBMUCOSA WITHIN NORMAL LIMITS WITH A MORPHOLOGICALLY BENIGN | ||
LYMPHOID NODULE. | |||
</pre> | </pre> | ||
==== | ====Suspected missed lesion==== | ||
<pre> | |||
RECTOSIGMOID, BIOPSY: | |||
- COLORECTAL-TYPE MUCOSA WITH A LYMPHOID AGGREGATE. | |||
- NEGATIVE FOR ACTIVE COLITIS. | |||
- NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY -- SEE COMMENT. | |||
COMMENT: | |||
The | The clinical history is noted. This biopsy does not show neoplastic tissue; | ||
however, the biopsy may not be representative of the lesion seen. | |||
Levels were cut and these did not yield additional information. There are | |||
no changes to suggest a chronic colitis. | |||
Correlation with imaging may be useful. A re-biopsy is suggested. | |||
</pre> | |||
====Micro - suspected IBD==== | |||
The sections show colorectal-type mucosa. The glands show no significant architectural | |||
abnormalities and mature normally to the surface. Rare apoptotic epithelial cells are seen. There is no cryptitis. Neutrophils are not apparent in the lamina propria. | |||
====Rare PMNs - no cryptitis==== | |||
The sections show colorectal mucosa with rare lymphoid aggregates. The architecture is | |||
within normal limits. The epithelium matures normally to the surface. Very rare neutrophils | |||
are present within the lamina propria. A very small number of crypts have one or two | |||
neutrophils. No definite cryptitis is present. | |||
==Fecal material== | |||
{{Main|Fecal material}} | |||
= | =Hyperplastic polyp= | ||
:''The [[stomach]] lesion is dealt with in [[hyperplastic polyp of the stomach]]''. | |||
{{Main|Hyperplastic polyp}} | |||
= | =Inflammatory pseudopolyp= | ||
{{Main|Inflammatory pseudopolyp}} | |||
=Adenomatous polyps= | =Adenomatous polyps= | ||
| Line 407: | Line 397: | ||
==Pseudoinvasion in colorectal adenomatous polyps== | ==Pseudoinvasion in colorectal adenomatous polyps== | ||
*[[AKA]] ''pseudoinvasion''. | *[[AKA]] ''pseudoinvasion''. | ||
*[[AKA]] ''epithelial misplacement''. | |||
* | {{Main|Pseudoinvasion in colorectal adenomatous polyps}} | ||
==High-risk features in (colorectal) adenomatous polyps with carcinoma== | ==High-risk features in (colorectal) adenomatous polyps with carcinoma== | ||
| Line 428: | Line 405: | ||
#[[Lymphovascular invasion]]. | #[[Lymphovascular invasion]]. | ||
#High-grade [[tumour budding]]. | #High-grade [[tumour budding]]. | ||
#*Tumour bud = 1-4 cell(s); "high-grade budding" is >=10 tumour buds in a field of 0.385 mm<sup>2</sup>.<ref name=pmid11952856>{{Cite journal | last1 = Ueno | first1 = H. | last2 = Murphy | first2 = J. | last3 = Jass | first3 = JR. | last4 = Mochizuki | first4 = H. | last5 = Talbot | first5 = IC. | title = Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer. | journal = Histopathology | volume = 40 | issue = 2 | pages = 127-32 | month = Feb | year = 2002 | doi = | PMID = 11952856 }}</ref> | #*Tumour bud = 1-4 cell(s); "high-grade budding" is >=10 tumour buds in a field of 0.385 mm<sup>2</sup>.<ref name=pmid11952856>{{Cite journal | last1 = Ueno | first1 = H. | last2 = Murphy | first2 = J. | last3 = Jass | first3 = JR. | last4 = Mochizuki | first4 = H. | last5 = Talbot | first5 = IC. | title = Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer. | journal = Histopathology | volume = 40 | issue = 2 | pages = 127-32 | month = Feb | year = 2002 | doi = | PMID = 11952856 }}</ref>‡ | ||
#**If the microscope has a 22 mm eye piece and... | #**If the microscope has a 22 mm eye piece and... | ||
#***A 20x objective, the field is approximately 0.950 mm<sup>2</sup> -- to match the area | #***A 20x objective, the field is approximately 0.950 mm<sup>2</sup> -- to match the buds/area -- it would be 24.68 buds/0.950 mm<sup>2</sup>. | ||
#***A 40x objective, the field is approximately 0.238 mm<sup>2</sup> -- to match the area | #***A 40x objective, the field is approximately 0.238 mm<sup>2</sup> -- to match the buds/area -- it would be 6.17 buds/0.238 mm<sup>2</sup>. | ||
#Extensive submucosal invasion. | #Extensive submucosal invasion. | ||
#*>= 4 mm width ''or'' >= 2 mm depth. | #*>= 4 mm width ''or'' >= 2 mm depth. | ||
If none of the above factors is present the risk of [[lymph node]] metastasis is < 1%. The presence of one risk factor increases the risk to ~20%. If multiple risk factors are present the chance of [[lymph node metastases]] is greater than 35%.<ref name=pmid15300569/> | If none of the above factors is present the risk of [[lymph node]] metastasis is < 1%. The presence of one risk factor increases the risk to ~20%. If multiple risk factors are present the chance of [[lymph node metastases]] is greater than 35%.<ref name=pmid15300569/> | ||
Note: | |||
*‡Tumour budding as per international consensus is now assessed in field area of 0.785 mm<sup>2</sup>.<ref name=pmid28548122>{{Cite journal | last1 = Lugli | first1 = A. | last2 = Kirsch | first2 = R. | last3 = Ajioka | first3 = Y. | last4 = Bosman | first4 = F. | last5 = Cathomas | first5 = G. | last6 = Dawson | first6 = H. | last7 = El Zimaity | first7 = H. | last8 = Fléjou | first8 = JF. | last9 = Hansen | first9 = TP. | title = Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. | journal = Mod Pathol | volume = 30 | issue = 9 | pages = 1299-1311 | month = Sep | year = 2017 | doi = 10.1038/modpathol.2017.46 | PMID = 28548122 }}</ref> | |||
==Traditional adenoma== | ==Traditional adenoma== | ||
:''Includes '''tubular adenoma''', '''tubulovillous adenoma''', and '''villous adenoma'''.'' | :''Includes '''tubular adenoma''', '''tubulovillous adenoma''', and '''villous adenoma'''.'' | ||
{{Main|Traditional adenoma}} | |||
{{Main| | |||
==Traditional serrated adenoma== | ==Traditional serrated adenoma== | ||
{{Main|Traditional serrated adenoma}} | |||
==Sessile serrated adenoma== | ==Sessile serrated adenoma== | ||
{{Main|Sessile serrated adenoma}} | |||
=Malignant polyps= | =Malignant polyps= | ||
| Line 682: | Line 432: | ||
===General=== | ===General=== | ||
* | *Diagnosis may be a challenging on a small biopsy. | ||
====Clinical==== | |||
Invasion can be predicted based on endoscopic findings: | |||
*[[Kudo pit pattern]].<ref name=pmid18458845>{{Cite journal | last1 = Onishi | first1 = T. | last2 = Tamura | first2 = S. | last3 = Kuratani | first3 = Y. | last4 = Onishi | first4 = S. | last5 = Yasuda | first5 = N. | title = Evaluation of the depth score of type V pit patterns in crypt orifices of colorectal neoplastic lesions. | journal = J Gastroenterol | volume = 43 | issue = 4 | pages = 291-7 | month = | year = 2008 | doi = 10.1007/s00535-008-2161-1 | PMID = 18458845 }}</ref> | |||
*Non-lifting sign.<ref name=pmid7926542>{{Cite journal | last1 = Uno | first1 = Y. | last2 = Munakata | first2 = A. | title = The non-lifting sign of invasive colon cancer. | journal = Gastrointest Endosc | volume = 40 | issue = 4 | pages = 485-9 | month = | year = | doi = | PMID = 7926542 }}</ref> | |||
**Presence predicts deeper invasion.<ref name=pmid10462651>{{Cite journal | last1 = Ishiguro | first1 = A. | last2 = Uno | first2 = Y. | last3 = Ishiguro | first3 = Y. | last4 = Munakata | first4 = A. | last5 = Morita | first5 = T. | title = Correlation of lifting versus non-lifting and microscopic depth of invasion in early colorectal cancer. | journal = Gastrointest Endosc | volume = 50 | issue = 3 | pages = 329-33 | month = Sep | year = 1999 | doi = 10.1053/ge.1999.v50.98591 | PMID = 10462651 }}</ref> | |||
===Microscopic=== | ===Microscopic=== | ||
One of the two following: | |||
#Dysplasia and evidence of invasion - features:<ref name=pmid22827760>{{Cite journal | last1 = Kimura | first1 = R. | last2 = Fujimori | first2 = T. | last3 = Ichikawa | first3 = K. | last4 = Ajioka | first4 = Y. | last5 = Ueno | first5 = H. | last6 = Ohkura | first6 = Y. | last7 = Kashida | first7 = H. | last8 = Togashi | first8 = K. | last9 = Yao | first9 = T. | title = Desmoplastic reaction in biopsy specimens of early colorectal cancer: a Japanese prospective multicenter study. | journal = Pathol Int | volume = 62 | issue = 8 | pages = 525-31 | month = Aug | year = 2012 | doi = 10.1111/j.1440-1827.2012.02840.x | PMID = 22827760 }}</ref> | |||
#*Nuclear changes seen in adenomatous polyps - malignant-appearing cells. | |||
#**Enlarged nuclei. | |||
#**Chromatin hyperchromatic ''or'' vesicular. | |||
*Nuclear changes seen in adenomatous polyps. | #**Round-shape ''or'' cigar-shaped and pseudostratified. | ||
**Enlarged. | #*Architectural changes - usually those of high-grade dysplasia: | ||
**Chromatin hyperchromatic ''or'' vesicular. | #**Cribriforming - most common. | ||
**Round- | #**Papillary tufting. | ||
*Architectural changes - usually those of high-grade dysplasia: | #**Budding. | ||
**Cribriforming. | #**Sheeting. | ||
**Papillary tufting. | #*Deep involvement - one of the two following - '''key feature''': | ||
**Budding. | #*#Malignant-appearing cells in the submucosa. | ||
#*#*Pseudoinvasion must be excluded. | |||
#*#[[Desmoplastic stromal response]]. | |||
#*#*Spindle cells with: | |||
#*#**Large nuclei (nucleus ~ size of a plasma cell). | |||
#*#**Eosinophilic cytoplasm. | |||
#[[Signet ring cell carcinoma|Signet ring cells]]. | |||
DDx: | DDx: | ||
*[[Pseudoinvasion]] - surrounded by lamina propria, desmoplasia lacking, hemosiderin-laden macrophages. | *[[Pseudoinvasion]] - surrounded by lamina propria, desmoplasia lacking, hemosiderin-laden macrophages. | ||
*[[Reactive changes]]. | |||
Note: | Note: | ||
*Desmoplastic response is ''not'' predictive of submucosal invasion in pedunculated polyps.<ref name=pmid20665053>{{Cite journal | last1 = Hirose | first1 = M. | last2 = Fukui | first2 = H. | last3 = Igarashi | first3 = Y. | last4 = Fujimori | first4 = Y. | last5 = Katake | first5 = Y. | last6 = Sekikawa | first6 = A. | last7 = Ichikawa | first7 = K. | last8 = Tomita | first8 = S. | last9 = Imura | first9 = J. | title = Detection of desmoplastic reaction in biopsy specimens is useful for predicting the depth of invasion of early colorectal cancer: a Japanese collaborative study. | journal = J Gastroenterol | volume = 45 | issue = 12 | pages = 1212-8 | month = Dec | year = 2010 | doi = 10.1007/s00535-010-0288-3 | PMID = 20665053 }} | *Desmoplastic response is ''not'' predictive of submucosal invasion in pedunculated polyps.<ref name=pmid20665053>{{Cite journal | last1 = Hirose | first1 = M. | last2 = Fukui | first2 = H. | last3 = Igarashi | first3 = Y. | last4 = Fujimori | first4 = Y. | last5 = Katake | first5 = Y. | last6 = Sekikawa | first6 = A. | last7 = Ichikawa | first7 = K. | last8 = Tomita | first8 = S. | last9 = Imura | first9 = J. | title = Detection of desmoplastic reaction in biopsy specimens is useful for predicting the depth of invasion of early colorectal cancer: a Japanese collaborative study. | journal = J Gastroenterol | volume = 45 | issue = 12 | pages = 1212-8 | month = Dec | year = 2010 | doi = 10.1007/s00535-010-0288-3 | PMID = 20665053 }} | ||
</ref> | </ref> | ||
====Image==== | |||
<gallery> | |||
Image:Cecal_adenocarcinoma.jpg | Colorectal carcinoma. (WC/Nephron) | |||
</gallery> | |||
===Sign out=== | ===Sign out=== | ||
| Line 710: | Line 478: | ||
RECTOSIGMOID TUMOUR, BIOPSY: | RECTOSIGMOID TUMOUR, BIOPSY: | ||
- INVASIVE ADENOCARCINOMA, MODERATELY DIFFERENTIATED. | - INVASIVE ADENOCARCINOMA, MODERATELY DIFFERENTIATED. | ||
</pre> | |||
<pre> | |||
RECTUM, BIOPSY: | |||
- INVASIVE ADENOCARCINOMA, MODERATELY DIFFERENTIATED. | |||
</pre> | |||
<pre> | |||
RECTUM, BIOPSY: | |||
- HIGHLY SUSPICIOUS FOR INVASIVE ADENOCARCINOMA, SEE MICROSCOPIC. | |||
- TUBULOVILLOUS ADENOMA WITH HIGH-GRADE DYSPLASIA. | |||
</pre> | </pre> | ||
| Line 716: | Line 495: | ||
There is cribriforming of glands and epithelial budding. Plump spindle cells with eosinophilic cytoplasm surround the abnormal epithelium (desmoplastic stroma). No definite submucosa is identified; the diagnosis is based on the stromal desmoplasia. | There is cribriforming of glands and epithelial budding. Plump spindle cells with eosinophilic cytoplasm surround the abnormal epithelium (desmoplastic stroma). No definite submucosa is identified; the diagnosis is based on the stromal desmoplasia. | ||
=====Suspicious===== | |||
The sections shows multiple fragments of colorectal-type mucosa with a tubule-forming and villous-forming epithelium that has cellular pseudostratification and enlarged hyperchromatic nuclei, from | |||
the crypt base to the luminal aspect (dysplasia). | |||
Cribriforming of glands is identified at multiple foci. Goblet cells are rare in the | |||
dysplastic epithelium. | |||
One fragment of tissue, measuring approximately 2 millimetres, has increased numbers of plump stromal cells (desmoplastic response); this is suspicious for invasive adenocarcinoma. | |||
=Hamartomatous polyps= | =Hamartomatous polyps= | ||
| Line 733: | Line 521: | ||
==Juvenile polyp== | ==Juvenile polyp== | ||
{{Main|Juvenile polyp}} | |||
==Peutz-Jeghers polyp== | ==Peutz-Jeghers polyp== | ||
{{Main|Peutz-Jeghers polyp}} | |||
==Cowden disease== | ==Cowden disease== | ||
| Line 837: | Line 549: | ||
==Cronkhite-Canada syndrome== | ==Cronkhite-Canada syndrome== | ||
*Abbreviated ''CCS''. | *Abbreviated ''CCS''. | ||
{{Main|Cronkhite-Canada syndrome}} | |||
==Ganglioneuroma== | ==Ganglioneuroma== | ||
| Line 860: | Line 560: | ||
**Large cells with a round nucleus and a prominent nucleolus. | **Large cells with a round nucleus and a prominent nucleolus. | ||
DDx: | |||
*[ | *[[Hyperplastic polyp with perineuromatous stroma]]. | ||
====Images==== | |||
<gallery> | |||
Image:Ganglioneuroma_-_intermed_mag.jpg | Ganglioneuroma - intermed. mag. (WC/Nephron) | |||
Image:Ganglioneuroma_-_high_mag.jpg | Ganglioneuroma - high mag. (WC/Nephron) | |||
Image:Ganglioneuroma_-_very_high_mag.jpg | Ganglioneuroma - very high mag. (WC/Nephron) | |||
</gallery> | |||
==Inflammatory myoglandular polyp== | ==Inflammatory myoglandular polyp== | ||
| Line 883: | Line 588: | ||
*Polypoid prolaping mucosal fold in [[diverticular disease]]. | *Polypoid prolaping mucosal fold in [[diverticular disease]]. | ||
*[[Inflammatory cloacogenic polyp]]. | *[[Inflammatory cloacogenic polyp]]. | ||
*Inflammatory cap polyp. | *[[Inflammatory cap polyp]]. | ||
Image: | Image: | ||
*[http://www.biomedcentral.com/1471-230X/10/10/figure/F3 IMP (biomedcentral.com)].<ref name=pmid20102635/> | *[http://www.biomedcentral.com/1471-230X/10/10/figure/F3 IMP (biomedcentral.com)].<ref name=pmid20102635/> | ||
==Leiomyoma== | |||
{{Main|Colonic leiomyoma}} | |||
{{Main|Leiomyoma}} | |||
*May present as a polyp in the colon.<ref name=pmid21915840>{{Cite journal | last1 = Kemp | first1 = CD. | last2 = Arnold | first2 = CA. | last3 = Torbenson | first3 = MS. | last4 = Stein | first4 = EM. | title = An unusual polyp: a pedunculated leiomyoma of the sigmoid colon. | journal = Endoscopy | volume = 43 Suppl 2 UCTN | issue = | pages = E306-7 | month = | year = 2011 | doi = 10.1055/s-0030-1256640 | PMID = 21915840 }}</ref> | |||
==Colonic polyp with reactive subepithelial cells== | |||
===Microscopic=== | |||
Features: | |||
*Surface epithelium with a reduced quantity of cytoplasm and less goblets (regenerative appearance). | |||
*Mildly atypical subepithelial cells with pale moderate-to-abundant cytoplasm and nuclear enlargement +/-nuclear hyperchromasia. | |||
===Sign out=== | |||
<pre> | |||
POLYP, ASCENDING COLON, POLYPECTOMY: | |||
- POLYPOID FRAGMENT OF COLONIC-TYPE MUCOSA WITH REACTIVE SUBEPITHELIAL | |||
CELLS, SEE COMMENT. | |||
- NEGATIVE FOR DYSPLASIA. | |||
COMMENT: | |||
A pankeratin and CK7 immunostains are non-concerning. A CD68 immunostain | |||
highlights lamina propria macrophages. | |||
</pre> | |||
=See also= | =See also= | ||
| Line 893: | Line 621: | ||
*[[Small bowel]]. | *[[Small bowel]]. | ||
*[[Colon]]. | *[[Colon]]. | ||
*[[Polypectomy]]. | |||
=References= | =References= | ||
Latest revision as of 23:51, 18 March 2018
Gastrointestinal tract polyps, also gastrointestinal polyps or GI polyps, are the bread & butter of a GI pathologists workload. Some of 'em are benign... some pre-malignant... some malignant... some weird. Most GI polyps are from the intestine, i.e. intestinal polyps.
Overview - there are four basic types:[1]
- Hyperplastic - harmless, most common - 90% of all colonic polyps.[2]
- Hamartomatous - weriod stuff, syndromic things.
- Inflammatory - think inflammatory bowel disease, AKA pseudopolyps.
- Adenomatous - premalignant, several types (see below).
Mnemonic: HHI-A.
Diagnostic variability for colorectal polyps is substantial among community pathologists.[3]
Basic approach
- Sessile (flat) or polypoid (spherical, possibly has a stalk)?
- Nuclear features of adenoma & loss of goblets (hyperchromatic nuclei, nuclei round vs. flat, loss of nuclear stratification)?
- Inflammation?
- Serrated architecture?
A set of decision trees for GI polyps
Decision tree - GI polyps
| GI polyp | |||||||||||||||||||||||||||||||||||||||
| Polypoid (Lollipop-like) | Sessile (flat) | ||||||||||||||||||||||||||||||||||||||
| Nuclear changes | No nuc. change | Serrated | Not serrated | ||||||||||||||||||||||||||||||||||||
| Polypoid adenoma (below) | Serrated | Not serrated | SSA versus HP | Normal versus VA | |||||||||||||||||||||||||||||||||||
| HP | See misc. polyps (below) | ||||||||||||||||||||||||||||||||||||||
Notes:
- Polypoid:
- Stalk visible (lollipop handle visible) or epithelial surface on three sides (or more).
- Sessile (flat):
- "Line of muscularis mucosa" visible +/- test tube-like intestinal crypts.
- Nuclear changes:
- Nuclear enlargement (elongation), crowding/pseudostratification, hyperchromasia (more blue) - especially at the surface, i.e. adjacent to the lumen (as opposed to the base of the crypt).
Decision tree - polypoid adenoma
| Polypoid adenoma | |||||||||||||||||||||||||||||||
| Serrated | Non-serrated | ||||||||||||||||||||||||||||||
| TSA | Tubular arch. | Tubulovillous arch. | Villous arch. | ||||||||||||||||||||||||||||
| TA | TVA | VA | |||||||||||||||||||||||||||||
Notes:[4]
- TA, tubular component >75%.
- VA, villous component >50%.
Decision tree - miscellaneous polyps
| Misc. polyps | |||||||||||||||||||||||||||||||
| Inflam. | No inflam. | ||||||||||||||||||||||||||||||
| Benign | Inflam. p. | Hamart. | Benign | ||||||||||||||||||||||||||||
| PJP | Juvenile | Other | |||||||||||||||||||||||||||||
Notes:
- Juvenile polyps may have marked inflammation.
Hamartomatous polyps - basic DDx:
- Juvenile polyp/Retention polyp -- DIES (dilated glands, incr. LP, eroded surface, stalk).
- Peutz-Jeghers polyp (PJP) - frond-like with all mucosa components .
"Other" includes diagnoses which require history or tissue surround the polyp. These include the polyps seen in:
Tabular comparison of colonic polyps
Overview in two tables
Common colonic polyps
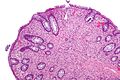
| Type | Key feature(s) | Details | Prevalence / prognosis | Other | DDx | Image |
|---|---|---|---|---|---|---|
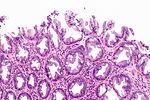
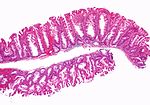
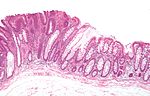
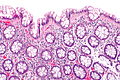
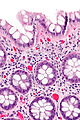
| Normal mucosa / no pathology | test tubes in a rack-like morphology | small nuclei, abundant goblet cells | common / benign | moderate inflammation is normal | missed lesion, colonic spirochetes, cryptosporidiosis, microscopic colitis, CMV colitis | |
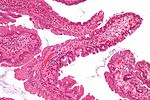
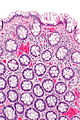
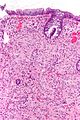
| Hyperplastic polyp | serrated at the surface | abundant goblet cells, usu. left colon; no features of SSA | common / benign | may be syndromic, e.g. hyperplastic polyposis syndrome | sessile serrated adenoma | |
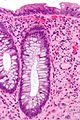
| Traditional adenoma | nuclear hyperchromasia & pseudostratification / crowding at the luminal aspect | decreased goblet cells, usu. polypoid - on a stalk, usu. left colon | common / premalignant | tubular adenoma, tubulovillous adenoma, villous adenoma | traditional serrated adenoma, reactive changes (inflammation) |
Less common
| Type | Key feature(s) | Details | Prevalence / prognosis | Other | DDx | Image |
|---|---|---|---|---|---|---|
| Sessile serrated adenoma (SSA) | basal crypt dilation & serration | boot-shaped crypts, horizontal crypts, branching crypts | uncommon / pre-malignant | AKA sessile serrated polyp | hyperplastic polyp | |
| Traditional serrated adenoma (TSA) | nuclear hyperchromasia & pseudostratification / crowding at the surface, serrated, villous-like architecture | decreased goblet cells | very rare / premalignant | called "traditional" to differentiate from SSA | traditional serrated adenoma (esp. villous adenoma) | |
| Juvenile polyp (retention polyp) | dilated glands, increased lamina propria | eroded surface (due to trauma), stalk (polypoid), inflammation - common | uncommon / benign if in isolation | may be part of juvenile polyposis syndrome | inflammatory pseudopolyp | |
| Inflammatory pseudopolyp | inflammation, erosion/ulceration adjacent to polyp | loss of mucosa adjacent to pseudopolyp | uncommon / seen in IBD, increased risk of malignancy | only seen in IBD; Dx implies IBD | juvenile polyp | |
| Peutz-Jeghers polyp (PJP) | branching smooth muscle | tree-like growth pattern | very rare / syndromic; assoc. with cancer | PJP not pre-malignant lesion in itself; see Peutz-Jeghers syndrome | normal, classically in the small bowel |
Common problems
Submucosal invasion
- This may be difficult to assess histomorphologically; these one should show a friend.
Pseudoinvasion
Early invasion
Adenomatous vs. hyperplastic
Adenomatous polyps & hyperplastic polyps - a comparison (adapted from Li and Burgart[5]):
| Attribute | Hyperplastic polyp (HP) | Sessile serrated adenoma (SSA) | Traditional serrated adenoma (TSA) | Traditional adenoma -tubular adenoma -tubulovillous adenoma -villous adenoma |
|---|---|---|---|---|
| Classic location | rectum/left colon | right colon | rectum/left colon | rectum/left colon |
| Morphology | polypoid | flat (sessile) | polypoid | polypoid |
| Cytologic atypia -Cigar nuclei -Hyperchromasia -Nuclear crowding |
absent | absent | present | present |
| Location of worst atypia | - | - | basal | luminal |
| Cytoplasm | eosinophilic | prominent eosinophilia | eosinophilic | basophilic |
| Goblet cells | abundant | common | less common | less common |
| Luminal Serration | present | common | present | absent |
| SSA architecture -Basal crypt serration -Basal crypt dilation -Horizonatal crypts -Branched crypts |
absent | present | absent | absent |
| Key feature(s) | serrated luminal surf. & goblets | abnorm. crypt arch. & sessile | nuclear atypia & serrated | nuclear atypia (luminal) |
| Image(s) |
Normal colonic mucosa:
- Nuclei - round and basally located.
- Abundant goblet cells.
- Moderate inflammation.
- Paneth cells - present in right colon.
- Glands - straight, no branching; "test tube" shape.
Notes: Left colon refers to the sigmoid colon, descending colon and the distal half of the transverse colon; right colon refers to the cecum, ascending colon and proximal half of the transverse colon.
Normal
Normal colorectal mucosa
General
- Endoscopists go after anything that is polypoid... and that may be normal.
Microscopic
Features:
- Test tube like glands.
- Minimal palisading.
- Nuclei <3:1 = height:width.
- No nuclear pseudostratification. †
- Deep part of crypt is more hyperchromatic than superficial component - important.
- The surface should be lighter staining than the deeper aspect, i.e. the deeper glands are dark blue and the superficial gland are light blue.
Note:
- † May be seen in reactive changes.
DDx (colorectal mucosa with minimal changes):
Images
www:
- Normal colorectal mucosa (uwa.edu.au).[6]
- Colon (siumed.edu).
- Normal colorectal mucosa (maricopa.edu).
Sign out
Normal
Cecum, Biopsy: - Colorectal-type mucosa within normal limits.
Right Colon, Biopsy: - Colonic mucosa within normal limits.
Transverse Colon, Biopsy: - Colonic mucosa within normal limits.
Left Colon, Biopsy: - Colonic mucosa within normal limits.
Rectum, Biopsy: - Colorectal mucosa within normal limits.
Block letters
SIGMOID COLON, BIOPSY: - COLORECTAL-TYPE MUCOSA WITHIN NORMAL LIMITS.
COLON, 70 CM, BIOPSY: - COLORECTAL-TYPE MUCOSA WITHIN NORMAL LIMITS.
Polypoid fragments
POLYP, SIGMOID COLON, BIOPSY: - POLYPOID FRAGMENT OF COLORECTAL-TYPE MUCOSA WITHIN NORMAL LIMITS.
Mucosa and submucosa
POLYP, SIGMOID COLON, BIOPSY: - COLONIC MUCOSA AND SUBMUCOSA WITHIN NORMAL LIMITS.
Lymphoid nodule present
- Lymphoid nodules manifest endoscopically as a small polypoid protuberances. It is worthwhile to report the presence of lymphoid nodules as they reassure the endoscopist that they probably sampled the abnormality they saw.
POLYP, RECTUM, BIOPSY: - RECTAL MUCOSA WITHIN NORMAL LIMITS WITH A MORPHOLOGICALLY BENIGN LYMPHOID AGGREGATE.
COLON, RIGHT SIDE, BIOPSY: - COLONIC MUCOSA WITH MORPHOLOGICALLY BENIGN LYMPHOID AGGREGATES, NO SIGNIFICANT PATHOLOGY.
Submucosa present
POLYP, ASCENDING COLON, BIOPSY: - COLONIC MUCOSA AND SUBMUCOSA WITHIN NORMAL LIMITS WITH A MORPHOLOGICALLY BENIGN LYMPHOID NODULE.
Suspected missed lesion
RECTOSIGMOID, BIOPSY: - COLORECTAL-TYPE MUCOSA WITH A LYMPHOID AGGREGATE. - NEGATIVE FOR ACTIVE COLITIS. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY -- SEE COMMENT. COMMENT: The clinical history is noted. This biopsy does not show neoplastic tissue; however, the biopsy may not be representative of the lesion seen. Levels were cut and these did not yield additional information. There are no changes to suggest a chronic colitis. Correlation with imaging may be useful. A re-biopsy is suggested.
Micro - suspected IBD
The sections show colorectal-type mucosa. The glands show no significant architectural abnormalities and mature normally to the surface. Rare apoptotic epithelial cells are seen. There is no cryptitis. Neutrophils are not apparent in the lamina propria.
Rare PMNs - no cryptitis
The sections show colorectal mucosa with rare lymphoid aggregates. The architecture is within normal limits. The epithelium matures normally to the surface. Very rare neutrophils are present within the lamina propria. A very small number of crypts have one or two neutrophils. No definite cryptitis is present.
Fecal material
Hyperplastic polyp
- The stomach lesion is dealt with in hyperplastic polyp of the stomach.
Inflammatory pseudopolyp
Adenomatous polyps
Overview
Several types of adenomatous polyps are recognized:
- Traditional adenomas (have three subtypes):
- Tubular adenoma - most common, lowest malignant potential.
- Tubulovillous adenoma.
- Villous adenoma - highest malignant potential.
- Sessile serrated adenomas:
- New kid on the block.
- Traditional serrated adenomas - nuclear features of 'traditional adenoma' + serrated architecture.
Notes:
- They are all considered pre-malignant, i.e. if you leave 'em in place they often develop into cancer.
- If multiple... think about familial adenomatous polyposis (FAP), attenuated FAP, MUTYH polyposis syndrome, serrated polyposis syndrome.
Management of (adenomatous colonic) polyps
Follow-up interval for polyps (colonoscopy interval):[7]
- Normal follow-up (includes presence of hyperplastic polyps): ~10 years.
- 1-2 low risk (adenomatous) polyps: 5-10 years.
- 3-10 low risk polyps or a high risk polyp: 3 years.
- >10 low risk polyps: <3 years.
- Inadequately removed polyps: <6 months.
Classified as high risk polyp (any of the following):[7]
- Tubulovillous.
- Villous.
- High grade dysplasia.
- Size >= 1 cm.
Mnemonic: GAS = grade (high), architecture (tubulovillous, villous), size (>1 cm).
Note:
- High risk polyp, as defined above, is also called advanced adenoma;[8] however, it should be noted that there are different definitions for advanced adenoma (e.g. Winawer & Zauber[9] include early invasive tumours). Thus, it is best to avoid the term.
Pseudoinvasion in colorectal adenomatous polyps
High-risk features in (colorectal) adenomatous polyps with carcinoma
Predictors of poor outcome with early submucosal invasion:[10]
- High tumour grade.
- Lymphovascular invasion.
- High-grade tumour budding.
- Tumour bud = 1-4 cell(s); "high-grade budding" is >=10 tumour buds in a field of 0.385 mm2.[11]‡
- If the microscope has a 22 mm eye piece and...
- A 20x objective, the field is approximately 0.950 mm2 -- to match the buds/area -- it would be 24.68 buds/0.950 mm2.
- A 40x objective, the field is approximately 0.238 mm2 -- to match the buds/area -- it would be 6.17 buds/0.238 mm2.
- If the microscope has a 22 mm eye piece and...
- Tumour bud = 1-4 cell(s); "high-grade budding" is >=10 tumour buds in a field of 0.385 mm2.[11]‡
- Extensive submucosal invasion.
- >= 4 mm width or >= 2 mm depth.
If none of the above factors is present the risk of lymph node metastasis is < 1%. The presence of one risk factor increases the risk to ~20%. If multiple risk factors are present the chance of lymph node metastases is greater than 35%.[10]
Note:
- ‡Tumour budding as per international consensus is now assessed in field area of 0.785 mm2.[12]
Traditional adenoma
- Includes tubular adenoma, tubulovillous adenoma, and villous adenoma.
Traditional serrated adenoma
Sessile serrated adenoma
Malignant polyps
Colorectal adenocarcinoma
General
- Diagnosis may be a challenging on a small biopsy.
Clinical
Invasion can be predicted based on endoscopic findings:
- Kudo pit pattern.[13]
- Non-lifting sign.[14]
- Presence predicts deeper invasion.[15]
Microscopic
One of the two following:
- Dysplasia and evidence of invasion - features:[16]
- Nuclear changes seen in adenomatous polyps - malignant-appearing cells.
- Enlarged nuclei.
- Chromatin hyperchromatic or vesicular.
- Round-shape or cigar-shaped and pseudostratified.
- Architectural changes - usually those of high-grade dysplasia:
- Cribriforming - most common.
- Papillary tufting.
- Budding.
- Sheeting.
- Deep involvement - one of the two following - key feature:
- Malignant-appearing cells in the submucosa.
- Pseudoinvasion must be excluded.
- Desmoplastic stromal response.
- Spindle cells with:
- Large nuclei (nucleus ~ size of a plasma cell).
- Eosinophilic cytoplasm.
- Spindle cells with:
- Malignant-appearing cells in the submucosa.
- Nuclear changes seen in adenomatous polyps - malignant-appearing cells.
- Signet ring cells.
DDx:
- Pseudoinvasion - surrounded by lamina propria, desmoplasia lacking, hemosiderin-laden macrophages.
- Reactive changes.
Note:
- Desmoplastic response is not predictive of submucosal invasion in pedunculated polyps.[17]
Image
Sign out
RECTOSIGMOID TUMOUR, BIOPSY: - INVASIVE ADENOCARCINOMA, MODERATELY DIFFERENTIATED.
RECTUM, BIOPSY: - INVASIVE ADENOCARCINOMA, MODERATELY DIFFERENTIATED.
RECTUM, BIOPSY: - HIGHLY SUSPICIOUS FOR INVASIVE ADENOCARCINOMA, SEE MICROSCOPIC. - TUBULOVILLOUS ADENOMA WITH HIGH-GRADE DYSPLASIA.
Micro
The sections shows colorectal-type mucosa with a tubule-forming epithelium that has cellular pseudostratification and enlarged hyperchromatic nuclei, from the crypt base to the luminal aspect (dysplasia).
There is cribriforming of glands and epithelial budding. Plump spindle cells with eosinophilic cytoplasm surround the abnormal epithelium (desmoplastic stroma). No definite submucosa is identified; the diagnosis is based on the stromal desmoplasia.
Suspicious
The sections shows multiple fragments of colorectal-type mucosa with a tubule-forming and villous-forming epithelium that has cellular pseudostratification and enlarged hyperchromatic nuclei, from the crypt base to the luminal aspect (dysplasia).
Cribriforming of glands is identified at multiple foci. Goblet cells are rare in the dysplastic epithelium.
One fragment of tissue, measuring approximately 2 millimetres, has increased numbers of plump stromal cells (desmoplastic response); this is suspicious for invasive adenocarcinoma.
Hamartomatous polyps
Overview
There are three well known hamartomatous polyp syndromes:[18]
There are two obscure hamartomatous polyp syndromes:[18]
- Bannayan-Riley-Ruvalcaba syndrome (BRBS).
- Devon polyposis syndrome (DPS).
Notes:
- BRBS is due to a PTEN mutation[19] (the same gene associated with Cowden's disease).
- DPS is reported in only one family that lives in Devon, UK.[20]
Juvenile polyp
Peutz-Jeghers polyp
Cowden disease
- AKA Cowden syndrome.
General
Etiology:
- PTEN gene mutation.
Clinical features:[21]
- Hamartomatous polyps.
- Facial trichilemmomas (hair follicle root sheath epithelium tumour).
- Oral papillomas.
- Acral keratoses (peripheral keratoses).
Note:
- Lame mnemonic PATH:[22] Papilloma (oral), Acral keratosis, Trichilemmoma, Hamartomatous polyps.
Microscopic
Features:
- Hamartomatous polyp - features non-specific. (???)
Weird stuff
Cronkhite-Canada syndrome
- Abbreviated CCS.
Ganglioneuroma
General
- May be part of MEN 2B.
Microscopic
Features - see ganglioneuroma:
- Ganglion cells - key feature.
- Large cells with a round nucleus and a prominent nucleolus.
DDx:
Images
Inflammatory myoglandular polyp
General
- Controversial - probably not a distinct pathologic entity.[23]
- Rare, benign, non-neoplastic.[24]
- Large bowel, usually rectosigmoid.
Microscopic
Features:[25]
- Granulation tissue within the lamina propria.
- Lamina propria smooth muscle.
- Irregular gland architecture:
- Cystic dilatation.
- Tortuosity.
DDx:[23]
- Mucosal prolapse syndrome.
- Polypoid prolaping mucosal fold in diverticular disease.
- Inflammatory cloacogenic polyp.
- Inflammatory cap polyp.
Image:
Leiomyoma
- May present as a polyp in the colon.[26]
Colonic polyp with reactive subepithelial cells
Microscopic
Features:
- Surface epithelium with a reduced quantity of cytoplasm and less goblets (regenerative appearance).
- Mildly atypical subepithelial cells with pale moderate-to-abundant cytoplasm and nuclear enlargement +/-nuclear hyperchromasia.
Sign out
POLYP, ASCENDING COLON, POLYPECTOMY: - POLYPOID FRAGMENT OF COLONIC-TYPE MUCOSA WITH REACTIVE SUBEPITHELIAL CELLS, SEE COMMENT. - NEGATIVE FOR DYSPLASIA. COMMENT: A pankeratin and CK7 immunostains are non-concerning. A CD68 immunostain highlights lamina propria macrophages.
See also
References
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 856. ISBN 0-7216-0187-1.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 858. ISBN 0-7216-0187-1.
- ↑ Rex, DK.; Alikhan, M.; Cummings, O.; Ulbright, TM. (Oct 1999). "Accuracy of pathologic interpretation of colorectal polyps by general pathologists in community practice.". Gastrointest Endosc 50 (4): 468-74. PMID 10502165.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 860. ISBN 0-7216-0187-1.
- ↑ Li SC, Burgart L (March 2007). "Histopathology of serrated adenoma, its variants, and differentiation from conventional adenomatous and hyperplastic polyps". Arch. Pathol. Lab. Med. 131 (3): 440-5. PMID 17516746. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=131&page=440.
- ↑ URL: http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/git.htm. Accessed on: 18 October 2012.
- ↑ 7.0 7.1 Levine JS, Ahnen DJ (December 2006). "Clinical practice. Adenomatous polyps of the colon". N. Engl. J. Med. 355 (24): 2551–7. doi:10.1056/NEJMcp063038. PMID 17167138. http://content.nejm.org/cgi/reprint/355/24/2551.pdf.
- ↑ Laiyemo, AO.; Murphy, G.; Albert, PS.; Sansbury, LB.; Wang, Z.; Cross, AJ.; Marcus, PM.; Caan, B. et al. (Mar 2008). "Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years.". Ann Intern Med 148 (6): 419-26. PMID 18347350.
- ↑ Winawer, SJ.; Zauber, AG. (Jan 2002). "The advanced adenoma as the primary target of screening.". Gastrointest Endosc Clin N Am 12 (1): 1-9, v. PMID 11916153.
- ↑ 10.0 10.1 Ueno, H.; Mochizuki, H.; Hashiguchi, Y.; Shimazaki, H.; Aida, S.; Hase, K.; Matsukuma, S.; Kanai, T. et al. (Aug 2004). "Risk factors for an adverse outcome in early invasive colorectal carcinoma.". Gastroenterology 127 (2): 385-94. PMID 15300569.
- ↑ Ueno, H.; Murphy, J.; Jass, JR.; Mochizuki, H.; Talbot, IC. (Feb 2002). "Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer.". Histopathology 40 (2): 127-32. PMID 11952856.
- ↑ Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, JF. et al. (Sep 2017). "Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016.". Mod Pathol 30 (9): 1299-1311. doi:10.1038/modpathol.2017.46. PMID 28548122.
- ↑ Onishi, T.; Tamura, S.; Kuratani, Y.; Onishi, S.; Yasuda, N. (2008). "Evaluation of the depth score of type V pit patterns in crypt orifices of colorectal neoplastic lesions.". J Gastroenterol 43 (4): 291-7. doi:10.1007/s00535-008-2161-1. PMID 18458845.
- ↑ Uno, Y.; Munakata, A.. "The non-lifting sign of invasive colon cancer.". Gastrointest Endosc 40 (4): 485-9. PMID 7926542.
- ↑ Ishiguro, A.; Uno, Y.; Ishiguro, Y.; Munakata, A.; Morita, T. (Sep 1999). "Correlation of lifting versus non-lifting and microscopic depth of invasion in early colorectal cancer.". Gastrointest Endosc 50 (3): 329-33. doi:10.1053/ge.1999.v50.98591. PMID 10462651.
- ↑ Kimura, R.; Fujimori, T.; Ichikawa, K.; Ajioka, Y.; Ueno, H.; Ohkura, Y.; Kashida, H.; Togashi, K. et al. (Aug 2012). "Desmoplastic reaction in biopsy specimens of early colorectal cancer: a Japanese prospective multicenter study.". Pathol Int 62 (8): 525-31. doi:10.1111/j.1440-1827.2012.02840.x. PMID 22827760.
- ↑ Hirose, M.; Fukui, H.; Igarashi, Y.; Fujimori, Y.; Katake, Y.; Sekikawa, A.; Ichikawa, K.; Tomita, S. et al. (Dec 2010). "Detection of desmoplastic reaction in biopsy specimens is useful for predicting the depth of invasion of early colorectal cancer: a Japanese collaborative study.". J Gastroenterol 45 (12): 1212-8. doi:10.1007/s00535-010-0288-3. PMID 20665053.
- ↑ 18.0 18.1 Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 345. ISBN 978-0443066573.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 153480
- ↑ Allibone, RO.; Nanson, JK.; Anthony, PP. (Jul 1992). "Multiple and recurrent inflammatory fibroid polyps in a Devon family ('Devon polyposis syndrome'): an update.". Gut 33 (7): 1004-5. PMID 1644320.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 858-9. ISBN 0-7216-0187-1.
- ↑ URL: http://www.pathologyexpert.com/boards/onlinefiles/syndromes.htm. Accessed on: 6 December 2011.
- ↑ 23.0 23.1 Bhathal, PS.; Chetty, R.; Slavin, JL. (Aug 1993). "Myoglandular polyps.". Am J Surg Pathol 17 (8): 852-3. PMID 8338196.
- ↑ 24.0 24.1 Meniconi, RL.; Caronna, R.; Benedetti, M.; Fanello, G.; Ciardi, A.; Schiratti, M.; Papini, F.; Farelli, F. et al. (2010). "Inflammatory myoglandular polyp of the cecum: case report and review of literature.". BMC Gastroenterol 10: 10. doi:10.1186/1471-230X-10-10. PMC 2828397. PMID 20102635. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2828397/.
- ↑ Nakamura, S.; Kino, I.; Akagi, T. (Aug 1992). "Inflammatory myoglandular polyps of the colon and rectum. A clinicopathological study of 32 pedunculated polyps, distinct from other types of polyps.". Am J Surg Pathol 16 (8): 772-9. PMID 1309176.
- ↑ Kemp, CD.; Arnold, CA.; Torbenson, MS.; Stein, EM. (2011). "An unusual polyp: a pedunculated leiomyoma of the sigmoid colon.". Endoscopy 43 Suppl 2 UCTN: E306-7. doi:10.1055/s-0030-1256640. PMID 21915840.
External links
- Serrated polyps quiz (unibas.ch) - nice quiz... though it is annoying that one has to click on the images to enlarge 'em.