Chondro-osseous tumours
Chondro-osseous tumours occasionally cross the desk of the pathologist. They are grouped together as bone may develop from cartilage.
Primary bone tumours are rare; the most common bone tumour is metastases.[1]
Bone tumours occasionally are lumped with soft tissue tumours. Soft tissue tumours are dealt with in the soft tissue lesions article. An introduction to bone is found in the bone article. An introduction to cartilage is found in the cartilage article.
General
- Diagnosis of a primary bone tumour should not be made without radiologic & clinical information!
- Metastasis:primary bone tumours = >20:1.[1]
Common malignant
- Osteosarcoma.
- Chondrosarcoma.
- Ewing's sarcoma.
- Multiple myeloma.
- Metastases.
- Most common tumours metastatic to bone (mnemonic: BLT with Ketchup & Pickles):
Epidemiology:[2]
- Osteosarcoma -> 2nd decade.
- Ewing's ->5-20 yrs.
- Chondrosarcoma -> from enchondroma or osteochrondroma -- patients over 40 yrs.
- Multiple myeloma -> most common primary bone tumour in adults.
Malignant bone tumours by age
Most common by age:[2]
- <1 year old - neuroblastoma.
- 1-10 years old - Ewing's of tubular bones.
- 10-30 years old - osteosarcoma, Ewing's of flat bones.
- 30-40 years old - reticulum cell sarcoma, fibrosarcoma, parosteal osteosarcoma, malignant giant cell tumour, lymphoma.
- >40 years old - mets, multiple myeloma, chondrosarcoma.
Benign aggressive bone tumours
- Giant cell tumour of bone.
- Osteoblastoma.
- Thought to be related to osteoid osteoma.
- If in long bones often diaphyseal.
Summary tables
Bone tumours
| Entity | Key feature | Other features | Radiology / gross | Clinical | Stains / other | Image |
|---|---|---|---|---|---|---|
| Osteoma | normal bone (???) | other features (???) | radiology / gross (???) | ? | no stains / may be assoc. with FAP | Image (???) |
| Osteoid osteoma | osteoblastic rimming | anastomosing bony trabeculae | <= 1.5 cm, metaphysis | painful, NSAIDs remove pain, young | IHC / other | |
| Osteoblastoma | osteoblastic rimming | anastomosing bony trabeculae | > 1.5 cm, metaphysis | not painful | IHC / other | |
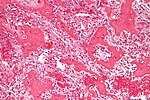
| Ewing sarcoma | small round blue cell tumour | cytoplasmic clearing (due to glycogen) | usu. diaphysis | pediatric, typically 1-10 years | PAS+, PASD- | 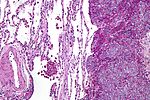 Ewing sarcoma. PAS stain. (WC) |
| Osteosarcoma | osteoid | +/-hemorrhage, +/-cartilage | distal femur, prox. tibia, prox. humerous | typically 10-30 years, pain, swelling | no stains; many subtypes | |
| Giant cell tumour of bone | abundant giant cells | nuclei of surrounding cells similar to those in giant cells | growth plate of long bones | 20-45 years old, +/-joint pain, +/-immobility | IHC / other |
Cartilage tumours
| Entity | Key feature | Other features | Radiology / gross | Clinical | Stains / other | Image |
|---|---|---|---|---|---|---|
| Chondroma | ctyologically benign cells | equally spaced nests | usu. diaphysis | benign / DDx: chondroma, well-diff. chondrosarcoma | IHC / bone marrow cavity chondroma = enchondroma | |
| Chondroblastoma | abundant extracellular material, abundant eosinophilic cytoplasm | calcifications surround cells nests ("chickenwire" appearance) - classic | epiphysis | DDx: giant cell tumour of bone | S100+ve, vimentin +ve | |
| Chondrosarcoma | cartilaginous appearance +/- nuclear atypia | lack osteoid, if present -> osteosarcoma | usu. diaphysis, classically hip; almost never distal extremity | >40 years old | IHC / may be histologically benign looking |
Other
| Entity | Key feature | Other features | Radiology / gross | Clinical | Stains / other | Image |
|---|---|---|---|---|---|---|
| Osteochondroma | benign bone and cartilage | Other features | metaphyseal lesions | Clinical | IHC / other | Image |
| Adamantinoma | bisphasic - stroma & epithelium | Other features | tibia, fibula, intracortical, radiolucent | Clinical | IHC / other | |
| Diffuse tenosynovial giant-cell tumour (AKA PVNS) | pigmented giant cells | nodules | Radiology / gross | Clinical | IHC / other | |
| Brown tumour | fibrosis, +/-giant cells | unaffected bone incr. osteoblasts and osteoclasts | Radiology / gross | due to hypercalcemia; not a neoplasm | IHC / other |
Radiology
Radiologic features
| Features | Benign | Malignant |
|---|---|---|
| Bone changes | sclerotic rim | tumour perforation |
| Circumscription | pushing margins | ill-defined/moth-eaten |
| Soft tissue involvement | no | common |
| Periosteal reaction | no | "hair-on-end" or "sunburst", "onion skin", Codman's triangle |
Location
| Diagnosis | Epiphysis | Metaphysis | Diaphysis | Type of lesion |
|---|---|---|---|---|
| Aneurysmal bone cyst | common | most common | rare | bone |
| Chondroblastoma | most common | rare | extremely rare | cartilage |
| Chondrosarcoma | uncommon | common | most common | cartilage |
| Chondromyxoid fibroma | rare | most common | common | other |
| Enchondroma | rare | common | common | cartilage |
| Ewing sarcoma | rare | common | most common | bone |
| Giant cell tumour | most common | rare | extremely rare | bone |
| Metastatic carcinoma | rare | common | most common | other |
| Non-ossifying fibroma | extremely rare | most common | common | other |
| Osteoblastoma | rare | most common | uncommon | bone |
| Osteochondroma | extremely rare[citation needed] | most common | common | bone/cartilage |
| Osteoid osteoma | uncommon | common | common[5] | bone |
| Osteosarcoma | rare | most common | uncommon | bone |
How to remember the primary bone lesions:
- Ewing sarcoma is the only malignant primary bone tumour of the diaphysis.
- Giant cell tumour of bone is the only primary bone lesion of the epiphysis.
- The rest of the primary bone lesions are metaphyseal.
- Osteochondroma is bone first and cartilage second. It behaves like most primary bone lesions; it is usually metaphyseal.
How to remember the primary cartilaginous lesions:
- Chondroblastoma is epiphyseal. The chicken wire goes around the chicken coop.
- The others are diaphyseal.
Cartilage
Chondroma
General
- Benign thingy.
- Usual legs and feet.
- May be difficult to separate from chondrosarcoma.
- Multiple chondromas = enchondromatosis; three distinct syndromes:[6]
- Ollier disease.
- Maffucci syndrome - with hemangiomas, increased risk of chondrosarcoma.[7]
- Metachondromatosis - autosomal dominant.
- Enchondroma = chondroma in the marrow space.
Clinical:[6]
- Pain.
Radiology
Features:[6]
- Lytic lesion.
- Usual close to a growth plate.
Microscopic
Features:
- Ctyologically benign cells is spaced nests.
Images:
Chondroblastoma
General
- Growth plate lesion.
- Sclerotic margin.
- "Young" = growth plates open.
- Typically painful.[8]
Gross
- Well-defined lesion.
Image:
Microscopic
Features:[9]
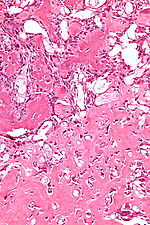
- Abundant extracellular material - pink on H&E stain - looks vaguely like cartilage.
- Chondroblasts:
- Nuclear morphology variable: ovoid, folded or grooved.
- Moderate-abundant eosinophilic cytoplasm.
- +/-Calcification surrounds the cell nests ("chickenwire" appearance) - classic feature.
- Cell nests have a thin pale blue rimming.
- +/-Giant cells.
- May lead to confusion with giant cell tumour of bone.
DDx:
- Giant cell tumour of bone.
- Chondroma.
- Well-differentiated chondrosarcoma.
Images:
- Chondroblastoma - intermed. mag. (WC).
- Chondroblastoma - very high mag. (WC).
- Chondroblastoma (medscape.com).[10]
- Chondroblastoma with "chickenwire" appearance (medscape.com).[10]
- Chondroblastoma (upmc.edu).[11]
IHC
Features:[9]
- S100 +ve.
- Vimentin +ve.[10]
Chondromyxoid fibroma
General
- Benign.
- Teenagers or young adults.
Gross
- Metaphyseal lesion.
- Well-circumscribed.
Microscopic
Features:[12]
- Spindle cells or stellate cells in a myxoid or chondroid stroma.
Images:
- Chondromyxoid fibroma - low mag. (webpathology.com).
- Chondromyxoid fibroma - high mag. (webpathology.com).
Chondrosarcoma
General
- Usually a good prognosis.
Clinical/epidemiologic features:[13]
- Usually arise in a (benign) abnormality of cartilage (e.g. osteochondroma, enchondroma).
- May be associated with a syndrome:
- Olier disease (multiple enchondromatosis).
- Maffucci syndrome (multiple enchondromas and hemangiomas).
Notes:
- Review article (from oncology perspective): PMID 17545802.
Subtypes
Several subtypes exist:
- Chondrosarcoma not otherwise specified (NOS).
- Juxtacortical chondrosarcoma.
- Myxoid chondrosarcoma.
- Mesenchymal chondrosarcoma.
- Clear cell chondrosarcoma.
- Dedifferentiated chondrosarcoma.
Microscopic
- "Abnormal cartilage":
- +/-Nuclear atypia - high grade lesions.
- High grade lesions:
- Nuclear clearing.
- Nucleoli.
- Hyperchromasia.
- Low/intermediate grade lesions:
- Bi-nucleation.
- Hypochromatic enlarged nuclei.
- Infiltration of lamellar bone ("invasion") - not common - diagnostic.
- High grade lesions:
- Increased cellularity.
- More cellular than cartilage... but relatively paucicellular compared to other sarcomas.
- Irregular spacing of chondrocytes.
- +/-Nuclear atypia - high grade lesions.
Notes:
- Low grade chondrosarcoma are not cytologically malignant; the diagnosis rests mostly on radiologic findings.
- The exception is infiltration of lamellar bone -- this is diagnostic of chondrosarcoma.[16]
DDx:
- Chordoma.
- Enchondroma.
- Synovial chondromatosis.
- Osteosarcoma - esp. chondroblastic osteosarcoma - has osteoid, may be focal.
Images:
- WC:
- www:
Variants
Mesenchymal chondrosarcoma
- Arise in soft tissue; this is where the name comes from.[17]
- Rare variant of chondrosarcoma.
Microscopic: Features:
- "White clouds in a blue sky".
Image:
Myxoid chondrosarcoma
Microscopic: Features:
DDx:
Extraskeletal myxoid chondrosarcoma
- Originally thought to be a variant of myxoid chondrosarcoma of bone; however, may not be a chondrosarcoma at all.[19]
- Characteristic chromosomal translocation: t(9;22) CHN-EWS.
DDx:
- Chordoma.[19]
- S-100 +ve (strong).
- EMA +ve.
Image:
Dedifferentiated chondrosarcoma
Clinical:
- Abysmal to poor prognosis.
Features:[22]
- Poorly differentiated (mesenchymal) malignancy.
- Well-differentiated cartilaginous component.
Images:
Grading
Features:[23]
- Grade I: mild-to-moderate increase of cellularity +/- binucleated cells.
- Grade II: between Grade I and Grade III.
- Grade III: nuclear pleomorphism, mitoses common.
IHC
- S-100 -ve. (???)
Bone
Osteoma
General
- Benign.
- May be associated with familial adenomatous polyposis.
- Normal bone - unusual location/outgrowth.
Microscopic
Features:
- Normal bone.
Osteoid osteoma
General
- Benign bone lesion.
Clinical:[24]
- Extremely painful.
- Relieved by NSAIDS.
Gross
Images:
Microscopic
Features:[24]
- Anastomosing bony trabeculae with:
- Variable mineralization.
- Mineralization (calcium phosphate) = purple on H&E stain.
- Osteoblasts rimming.
- Cells line-up at edge of bone.
- Variable mineralization.
Note:
- Histomorphologically near identical/indistinguishable from osteoblastoma.[28]
Images:
- Osteoid osteoma - CT scan (med.utah.edu).
- Osteoid osteoma (sciencephoto.com).
- Osteoid osteoma - low mag. (WC).
- Osteoid osteoma - high mag. (WC).
Osteoblastoma
General
- Benign bone tumour.
Microscopic
Features:[24]
- Anastomosing bony trabeculae with:
- Osteoblasts rimming.
- Cells line-up at edge of bone.
- Osteoblasts rimming.
Notes:
- Histomorphologically near identical/indistinguishable from osteoid osteoma.[28]
- Must be greater 1.5 cm by definition.[28]
Images:
Ewing sarcoma
- AKA EWS/pPNET, AKA (confusingly) EWS/PNET:
- EWS = Ewing sarcoma.
- pPNET = peripheral primitive neuroectodermal tumour.
- EWS and pPNET were once thought to be different tumours.
Note:
- Peripheral primitive neuroectodermal tumour should not be confused with primitive neuroectodermal tumour, commonly abbreviated PNET, a (supertentorial) brain tumour with similarities to medulloblastoma.
General
Clinical:
- Painful.
- Usually younger than 20 years.
- Second most common malignant bone tumour in children.
- Most common malignant bone tumour = osteosarcoma (AKA osteogenic sarcoma).
Poor prognostic factors:[29]
- Age (18 years-old+).
- Pelvis (extremity = good).
- >8 cm.
- Metastases.
- EWS-FL1 fusion type 2.
- >90% necrosis.
Etiology:
- Unknown origin; hypothesis: Ewing sarcoma arises from mesenchymal stem cell.[30]
Radiology
Features:[31]
- Long bones, diaphyses.
- Destructive.
- "Onion-skin" periosteal reaction.
Microscopic
Features:[32]
- Scant clear cytoplasm (contain glycogen -- PAS +ve, PAS-D -ve) - key feature.
- Round small nucleus.
- Usu. lack nucleoli.
- Usu. minimal-moderate size variation.
- Mitoses (common).
Notes:
- It is a small round cell tumour.
- Rhabdomyosarcoma occasionally has cytoplasmic clearing (due to glycogen).[33]
Images:
- WC:
- www:
IHC
Features:[34]
- CD99 +ve -- 1. diffuse, 2. plasma membrane staining; both required -- most specific.
- FLI-1 +ve.[35]
- CD45 -ve.
- Done to r/o lymphoma.
- +/-Neural markers (NSE, synaptophysin, CD57 (??? CD56 ???), S100).
- +/-Cytokeratins.
- Caveolin-1 +ve in ~ 85% of EWS.[36]
- WT-1 -ve.[37]
Notes:[38]
- CD99 +ve
- Plasma membrane staining tumours:
- Weak/cytoplasmic staining:
- FLI-1 +ve:[35]
Molecular diagnostics
Common features:
- EWS/FLI-1 fusion gene formation due to translocation: t(11;22)(q24;q12).[39][40]
- Often detected by RT-PCR (with EWS 5' and FLI-1 3' primers).
- Type 1 = EWS exon 7 + FLI-1 exon 6; good prognosis.
- Type 2 = others; poor prognosis.
Notes:
- The t(11;22)(q24;q12) is seen in ~90% of EWS/PNET... but also in:
- Olfactory neuroblastoma.
- Small cell osteogenic sarcoma (small cell variant of osteosarcoma).
- Polyphenotypic tumours.
- Rhabdomyosarcoma.
- Neuroblastoma (possibly).
- Several other EWS translocations exist.[41]
- ERG,[42] ETV1, E1AF and FEV.
- Lack of molecular findings does not exclude Ewing sarcoma.
- Testing:
- A break apart probe for EWS is a common way to look for pathologic change, as it covers almost all variants.
Electron microscopy
- Primitive cell junctions.
- Clear zone (glycogen lakes).
Osteosarcoma
- AKA osteogenic sarcoma.
General
- Most common malignant bone tumour in children.
- May be seen in the context of Li-Fraumeni syndrome.
Trivia:
- Terry Fox was afflicited by this tumour.
Definition
- Tumour that makes osteoid.
- Osteoid = (extracellular) organic component of bone, normally produced by osteoblasts (cells which make bone matrix).
Gross
Classic locations:[43]
- Distal femur ~ 45%.
- Proximal tibia ~ 20%.
- Proximal humerous ~ 15%.
Microscopic
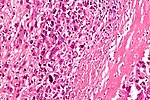
Features:
- Cells with malignant features (e.g. nuclear membrane irregularities, marked nuclear size differences, mitoses) surrounded by delicate strands of osteoid.
- Osteoid on H&E: pink, homogenous, "glassy".
- Tumours typically very cellular - when compared to normal bone.
- Large (multinucleated) osteoclast-like giant cells may be seen.[44]
Images:
- Osteosarcoma - intermed. mag. (WC).
- Osteosarcoma - very high mag. (WC).
- Small cell osteosarcoma - intermed. mag. (WC).
- Small cell osteosarcoma - high mag. (WC).
Subtypes
- Conventional osteosarcoma (high grade).
- Osteoblastic osteosarcoma.
- Fibroblastic osteosarcoma.
- Chondroblastic osteosarcoma.
- Small cell osteosarcoma.
- Telangiectatic osteosarcoma.
- Parosteal osteosarcoma.
- Periosteal osteosarcoma.
- Low-grade central osteosarcoma.
- High-grade surface osteosarcoma.
- Secondary osteosarcoma.
- Gnathic osteosarcoma - jaw bones - usually chondroblastic.
How to remember:
- Convention FOC = fibroblastic, osteogenic, chondroblastic.
- Low-grade central.
- High-grade surface.
- Parosteal.
- Periosteal.
- Small cell.
- Secondary.
- Telangiectatic.
Chondroblastic osteosarcoma
- Chondroid matrix present - may be prominent; osteoid may be a minor component.
- May be confused with chondrosarcoma.
Fibroblastic osteosarcoma
- Undifferentiated pleomorphic sarcoma-like/MFH-like.
Images:
Low-grade central osteosarcoma
- Well-formed bone.
- Usu. minimal nuclear atypia.
DDx:
Telangiectatic osteosarcoma
Important radiologic DDx:
Parosteal osteosarcoma
DDx:
Periosteal osteosarcoma
- Intermediate grade.[51]
Small cell osteosarcoma
- May mimic (other) small round cell tumours.
Secondary osteosarcoma
Arises in the context of something else - causes:
- Paget disease of the bone (~80% of secondary osteosarcomas)
- Radiation (~15% of secondary osteosarcomas)).[52]
- Prognosis often poor.[51]
Images:
Giant cell tumour of bone
General
Features:[53]
- Approximately 5% of primary bone tumours.
- Typical age: 20-45 years.
Clinical
- Location: growth plate of long bones.[54]
- May present with joint pain, immobility.
Note:
- Several types of giant cell tumours exist.
Microscopic
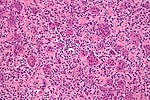
Features:[55]
- Giant cells with a large number of nuclei (usu. >10 in the plane of section).
- Usu. have prominent nucleoli.
- Mononuclear cells and small multinucleated cells with nuclei similar to those in the giant cells - key feature.
- +/-Hemosiderin deposition - not common.[56][57]
Notes:
- Giant cells typically present in abundance.
DDx:
- Giant cell lesions.
- Aneurysmal bone cyst - typically has spindle cells around the giant cells.
Images:
IHC
Other
This section collects stuff that doesn't neatly fit into the bone or cartilage category.
Osteochondroma
General
- Benign.
- Very common.
- Abnormal outgrowth of bone and cartilage - associated with growth plate.
- Usually present before age 20.[60]
Gross
Features:
- Metasphyseal lesions.[61]
- Mushroom-like shape - with broad or narrow base.
Note:
- Very unusual in craniofacial bones.[60]
Microscopic
Features:[60]
- Bone - forms base of "mushroom".
- Cartilage - layer usu. thinner in older individuals.
- Lobular arrangement[63] - clusters of 3-12 lacunae (separated by stroma).
- May have mild atypia.
- Nuclear enlargement - up to 5x normal.
- Perichondrium - covers the cartilage.
- Thin layer of cells - higher cellular density than cartilage.
- Bland spindle cells in a fibrous (eosinophilic) stroma.
- Thin layer of cells - higher cellular density than cartilage.
Notes:
- Benign cartilage - one chondrocyte per lacuna, small and round nucleus, no binucleation.
DDx:
- Chondrosarcoma - esp. in older individuals.
Images:
Diffuse tenosynovial giant-cell tumour
- AKA tenosynovial giant-cell tumour, diffuse type.
- Previously known as pigmented villonodular synovitis (PVNS).[64]
General
- Course: benign.
- Giant cell tumor of the tendon sheath is considered to be the soft-tissue counterpart of PVNS.[65]
Microscopic
Features:[66]
- Subsynovial nodules composed of cells with:
- Abundant cytoplasm.
- Pale nuclei.
- Multinucleated giant cells.
- Hemosiderin-laden macrophages.
- Foam cells.
Images:
Giant cell tumour of tendon sheath
- Abbreviated GCT of tendon sheath.
General
- Can be thought of as the small joint version of diffuse tenosynovial giant-cell tumour (AKA PVNS).[67]
- Rarely recur.
- Classically afflicts the hand.[68]
- Rarely malignant.[69][70]
Gross
Features:[68]
- Circumscribed mass - yellow-brown to tan.
Note:
- May be associated with bony erosions in larger lesions.[68]
Image:
Microscopic
Features:[67]
- Foam cells.
- Cells with moderate to abundant foamy-appearing cytoplasm.
- Multinucleated giant cells - may be scarce.
- +/-Tendon.
- Dense connective tissue.
- +/-Hemosiderin-laden macrophages.
Note:
- Features of malignancy: nuclear pleomorphism,[70] abnormal mitoses, >10 mitoses/HPF, tumour necrosis lack of maturation to superficial part (nuclei shrink, cytoplasm lipid-ified).[67]
DDx:
Images
www:
- GCT of tendon sheath - very low mag. (webpathology.com)
- GCT of tendon sheath - low mag. (webpathology.com).
- GCT of tendon sheath - high mag. (webpathology.com).
Sign out
LESION, RIGHT INDEX FINGER, EXCISION: - GIANT CELL TUMOUR OF THE TENDON SHEATH.
Micro
The sections show histiocytes and rare multinucleated giant cells on a background of dense connective tissue compatible with tendon. No nuclear atypia is apparent. Rare mitotic activity is identified. No atypical mitoses are apparent.
Adamantinoma
- Should not be confused with adenomatoid tumour.
General
Features:[31]
- Rare: < 1% of bone tumours.
- 25-35 years old.
- Tibia, fibula.
- Benign, may be locally aggressive.
- Cousin of ameloblastoma. (???)
Radiology
- Intracortical, radiolucent.
Microscopic
Features:
- Biphasic tumour:
- Fibrous/spindle cell component.
- Epithelial component.
Images:
DDx:[73]
- Vascular tumours (Epithelioid hemangioendothelioma).
- Metastatic carcinoma.
IHC
Features:[73]
- CK14 +ve (HMWK).[74]
- CK19 +ve (LMWK).
- CK8/18 -ve (LMWK).
Brown tumour
General
- Not a true neoplasm.[75]
- If tumour is understood as a synonym for neoplasm, the name is a misnomer.
- May (clinically) mimic a true neoplasm.
- Due to hyperparathyroidism - usually parathyroid adenoma.
- Usually secondary to chronic renal failure.
Hypercalcemia DDx
Mnemonic GRIMED:[76]
- Granulomatous disease (tuberculosis, sarcoidosis).
- Renal disease.
- Immobility.
- Malignancy (esp. squamous cell carcinoma, plasmacytoma).
- Endocrine (primary hyperparathyroidism - leads to brown tumour).
- Drugs (thiazides ... others).
Microscopic
Features:
- Fibrosis.
- +/-Giant cells with round to oval nuclei and nucleoli.[77]
- Bone unaffected by tumour - increased numbers of the following:
- Multinucleated cells (osteoclasts).
- Mononuclear cells around the bony trabeculae (osteoblasts).
DDx:
- Giant cell tumour of bone and other giant cell lesions.
Images:
See also
References
- ↑ 1.0 1.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 632. ISBN 978-0781765275.
- ↑ 2.0 2.1 Yeung, J.C.; Leonard, Blair J. N. (2005). The Toronto Notes 2005 - Review for the MCCQE and Comprehensive Medical Reference (2005 ed.). The Toronto Notes Inc. for Medical Students Inc.. pp. OR42. ISBN 978-0968592854.
- ↑ Yeung, J.C.; Leonard, Blair J. N. (2005). The Toronto Notes 2005 - Review for the MCCQE and Comprehensive Medical Reference (2005 ed.). The Toronto Notes Inc. for Medical Students Inc.. pp. OR41. ISBN 978-0968592854.
- ↑ URL: http://www.emedicine.com/RADIO/topic494.htm.
- ↑ URL: http://www.wheelessonline.com/ortho/osteoid_osteoma. Accessed on: 7 May 2012
- ↑ 6.0 6.1 6.2 URL: http://emedicine.medscape.com/article/389224-overview. Accessed on: 25 December 2010.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 166000
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 625. ISBN 978-1416054542.
- ↑ 9.0 9.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 642. ISBN 978-0781765275.
- ↑ 10.0 10.1 10.2 URL: http://emedicine.medscape.com/article/1254949-diagnosis. Accessed on: 31 December 2010.
- ↑ URL: http://path.upmc.edu/cases/case494.html. Accessed on: 24 January 2012.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 642. ISBN 978-0781765275.
- ↑ Skubitz KM, D'Adamo DR (November 2007). "Sarcoma". Mayo Clin. Proc. 82 (11): 1409–32. PMID 17976362. http://www.mayoclinicproceedings.com/content/82/11/1409.long.
- ↑ IAV. 26 February 2009.
- ↑ Klatt, Edward C. (2006). Robbins and Cotran Atlas of Pathology (1st ed.). Saunders. pp. 417. ISBN 978-1416002741.
- ↑ Dickson, B. 28 April 2011.
- ↑ Dowling EA (June 1964). "Mesenchymal chondrosarcoma". J Bone Joint Surg Am 46: 747–54. PMID 14161087. http://www.ejbjs.org/cgi/reprint/46/4/747.pdf.
- ↑ Fisher C (May 2000). "Parachordoma exists--but what is it?". Adv Anat Pathol 7 (3): 141–8. PMID 10809219.
- ↑ 19.0 19.1 Aigner, T.; Oliveira, AM.; Nascimento, AG. (Feb 2004). "Extraskeletal myxoid chondrosarcomas do not show a chondrocytic phenotype.". Mod Pathol 17 (2): 214-21. doi:10.1038/modpathol.3800036. PMID 14657948.
- ↑ URL: http://www.cttr.org/cms/?p=736. Accessed on: 1 May 2011.
- ↑ Mitchell, AD.; Ayoub, K.; Mangham, DC.; Grimer, RJ.; Carter, SR.; Tillman, RM. (Jan 2000). "Experience in the treatment of dedifferentiated chondrosarcoma.". J Bone Joint Surg Br 82 (1): 55-61. PMID 10697315.
- ↑ 22.0 22.1 Sopta, J.; Dordević, A.; Tulić, G.; Mijucić, V. (Feb 2008). "Dedifferentiated chondrosarcoma: our clinico-pathological experience and dilemmas in 25 cases.". J Cancer Res Clin Oncol 134 (2): 147-52. doi:10.1007/s00432-007-0262-5. PMID 17653766.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 643. ISBN 978-0781765275.
- ↑ 24.0 24.1 24.2 Mills, Stacey E; Carter, Darryl; Greenson, Joel K; Oberman, Harold A; Reuter, Victor E (2004). Sternberg's Diagnostic Surgical Pathology (4th ed.). Lippincott Williams & Wilkins. pp. 285. ISBN 978-0781740517.
- ↑ 25.0 25.1 URL: http://radiology.uthscsa.edu/CME/ELTXT/OOT/skeletallocation.html http://radiology.uthscsa.edu/CME/ELTXT/OOT/skeletallocation.html]. Accessed on: 7 May 2012.
- ↑ URL: http://www.radiologyassistant.nl/en/494e15cbf0d8d. Accessed on: 7 May 2012.
- ↑ URL: http://njms2.umdnj.edu/tutorweb/gross.htm. Accessed on: 7 May 2012.
- ↑ 28.0 28.1 28.2 Mills, Stacey E; Carter, Darryl; Greenson, Joel K; Oberman, Harold A; Reuter, Victor E (2004). Sternberg's Diagnostic Surgical Pathology (4th ed.). Lippincott Williams & Wilkins. pp. 286. ISBN 978-0781740517.
- ↑ PST. 14 February 2011.
- ↑ Lin PP, Wang Y, Lozano G (2011). "Mesenchymal Stem Cells and the Origin of Ewing's Sarcoma". Sarcoma 2011. doi:10.1155/2011/276463. PMC 2952797. PMID 20953407. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2952797/.
- ↑ 31.0 31.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 650. ISBN 978-0781765275.
- ↑ PST. 22 February 2010.
- ↑ PST. 14 February 2011.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 651. ISBN 978-0781765275.
- ↑ 35.0 35.1 Rossi S, Orvieto E, Furlanetto A, Laurino L, Ninfo V, Dei Tos AP (May 2004). "Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody". Mod. Pathol. 17 (5): 547–52. doi:10.1038/modpathol.3800065. PMID 15001993. http://www.nature.com/modpathol/journal/v17/n5/full/3800065a.html.
- ↑ PST. 14 February 2011.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 286. ISBN 978-0781765275.
- ↑ PST. 22 February 2010.
- ↑ URL: http://atlasgeneticsoncology.org/Tumors/Ewing5010.html. Accessed on: 23 February 2010.
- ↑ Turc-Carel C, Aurias A, Mugneret F, et al. (June 1988). "Chromosomes in Ewing's sarcoma. I. An evaluation of 85 cases of remarkable consistency of t(11;22)(q24;q12)". Cancer Genet. Cytogenet. 32 (2): 229–38. PMID 3163261.
- ↑ URL: http://www.cancerindex.org/geneweb/EWSR1.htm. Accessed on: 20 November 2011.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 165080
- ↑ Greenwald, J.; Heng, M. (2007). Toronto Notes for Medical Students 2007 (2007 ed.). The Toronto Notes Inc. for Medical Students Inc.. pp. OR43. ISBN 978-0968592878.
- ↑ Papalas JA, Balmer NN, Wallace C, Sangueeza OP (June 2009). "Ossifying dermatofibroma with osteoclast-like giant cells: report of a case and literature review". Am J Dermatopathol 31 (4): 379-83. doi:10.1097/DAD.0b013e3181966747. PMID 19461244.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 638. ISBN 978-0781765275.
- ↑ URL: http://bestpractice.bmj.com/best-practice/monograph/780/basics/classification.html. Accessed on: 7 April 2011.
- ↑ Inwards, CY (2001). "Low-grade central osteosarcoma versus fibrous dysplasia". Pathology Case Reviews 6 (1): 22-27. http://journals.lww.com/pathologycasereviews/Fulltext/2001/01000/Low_Grade_Central_Osteosarcoma_Versus_Fibrous.5.aspx.
- ↑ Patibandla, MR.; Uppin, SG.; Thotakura, AK.; Panigrahi, MK.; Challa, S.. "Primary telangiectatic osteosarcoma of occipital bone: a case report and review of literature.". Neurol India 59 (1): 117-9. doi:10.4103/0028-3886.76891. PMID 21339678.
- ↑ Weiss, A.; Khoury, JD.; Hoffer, FA.; Wu, J.; Billups, CA.; Heck, RK.; Quintana, J.; Poe, D. et al. (Apr 2007). "Telangiectatic osteosarcoma: the St. Jude Children's Research Hospital's experience.". Cancer 109 (8): 1627-37. doi:10.1002/cncr.22574. PMID 17351949.
- ↑ The International Agency for Research on Cancer (Editors: Fletcher, C.D.M.; Unni, K. Krishnan; Mertens, F.) (2006). Pathology and Genetics of Tumours of Soft Tissue and Bone (IARC WHO Classification of Tumours) (3rd ed.). World Health Organization. pp. 279. ISBN 978-9283224136.
- ↑ 51.0 51.1 51.2 Carrle, D.; Bielack, SS. (Dec 2006). "Current strategies of chemotherapy in osteosarcoma.". Int Orthop 30 (6): 445-51. doi:10.1007/s00264-006-0192-x. PMC 3172747. PMID 16896870. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3172747/.
- ↑ URL: http://www.rsna.org/REG/publications/rg/afip/privateM/1997/0017/0005/1205/6.htm. Accessed on: 8 April 2011.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 648. ISBN 978-0781765275.
- ↑ Wülling, M.; Engels, C.; Jesse, N.; Werner, M.; Delling, G.; Kaiser, E. (Aug 2001). "The nature of giant cell tumor of bone.". J Cancer Res Clin Oncol 127 (8): 467-74. PMID 11501745.
- ↑ Klatt, Edward C. (2006). Robbins and Cotran Atlas of Pathology (1st ed.). Saunders. pp. 420. ISBN 978-1416002741.
- ↑ Aoki, J.; Moriya, K.; Yamashita, K.; Fujioka, F.; Ishii, K.; Karakida, O.; Imai, S.; Sakai, F. et al. "Giant cell tumors of bone containing large amounts of hemosiderin: MR-pathologic correlation.". J Comput Assist Tomogr 15 (6): 1024-7. PMID 1939753.
- ↑ Matsushige, T.; Nakaoka, M.; Yahara, K.; Kagawa, K.; Miura, H.; Ohnuma, H.; Kurisu, K. (Aug 2008). "Giant cell tumor of the temporal bone with intratumoral hemorrhage.". J Clin Neurosci 15 (8): 923-7. doi:10.1016/j.jocn.2007.03.013. PMID 18554912.
- ↑ Dickson BC, Li SQ, Wunder JS, et al. (April 2008). "Giant cell tumor of bone express p63". Mod. Pathol. 21 (4): 369–75. doi:10.1038/modpathol.2008.29. PMID 18311114.
- ↑ Alberghini M, Kliskey K, Krenacs T, et al. (January 2010). "Morphological and immunophenotypic features of primary and metastatic giant cell tumour of bone". Virchows Arch. 456 (1): 97–103. doi:10.1007/s00428-009-0863-2. PMID 20012988.
- ↑ 60.0 60.1 60.2 60.3 Parkin, B.; Bennett, JC.; Zaher, A. (Aug 2003). "Pathologic quiz case: an iliac mass in a 25-year-old woman. Osteochondroma.". Arch Pathol Lab Med 127 (8): e355-6. doi:10.1043/1543-2165(2003)127e355:PQCAIM2.0.CO;2. PMID 12873205.
- ↑ URL: http://www.medpath.info/MainContent/Skeletal/Bone_07.html. Accessed on: 18 September 2012.
- ↑ Kokavec, M.; Gajdoš, M.; Džupa, V. (2011). "[Osteochondroma of the iliac crest: case report].". Acta Chir Orthop Traumatol Cech 78 (6): 583-5. PMID 22217414.
- ↑ 63.0 63.1 URL: http://path.upmc.edu/cases/case341/dx.html. Accessed on: 19 September 2012.
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1247. ISBN 978-1416031215.
- ↑ URL: http://emedicine.medscape.com/article/1253223-overview. Accessed on: 6 January 2011.
- ↑ URL: http://www.wheelessonline.com/ortho/pigmented_villonodular_synovitis.
- ↑ 67.0 67.1 67.2 Tadrous, Paul.J. Diagnostic Criteria Handbook in Histopathology: A Surgical Pathology Vade Mecum (1st ed.). Wiley. pp. 341. ISBN 978-0470519035.
Cite error: Invalid
<ref>tag; name "Ref_DCHH341" defined multiple times with different content Cite error: Invalid<ref>tag; name "Ref_DCHH341" defined multiple times with different content - ↑ 68.0 68.1 68.2 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 612. ISBN 978-0781765275.
- ↑ Pan, YW.; Huang, XY.; You, JF.; Tian, GL.; Li, C. (Nov 2008). "[Malignant giant cell tumor of the tendon sheaths in the hand].". Zhonghua Wai Ke Za Zhi 46 (21): 1645-8. PMID 19094761.
- ↑ 70.0 70.1 Shinjo, K.; Miyake, N.; Takahashi, Y. (Oct 1993). "Malignant giant cell tumor of the tendon sheath: an autopsy report and review of the literature.". Jpn J Clin Oncol 23 (5): 317-24. PMID 8230758.
- ↑ Suresh, SS.; Zaki, H. (Dec 2010). "Giant cell tumor of tendon sheath: case series and review of literature.". J Hand Microsurg 2 (2): 67-71. doi:10.1007/s12593-010-0020-9. PMID 22282671.
- ↑ URL: http://southbaypath.org/CaseImages/sb5260/sb5260.htm. Accessed on: 7 December 2010.
- ↑ 73.0 73.1 URL: http://www.pathconsultddx.com/pathCon/diagnosis?pii=S1559-8675%2806%2970057-2. Accessed on: 28 April 2011.
- ↑ URL: http://www.nordiqc.org/Epitopes/Cytokeratins/cytokeratins.htm. Accessed on: 28 April 2011.
- ↑ Meydan N, Barutca S, Guney E, et al. (June 2006). "Brown tumors mimicking bone metastases". J Natl Med Assoc 98 (6): 950–3. PMC 2569361. PMID 16775919. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2569361/?page=1.
- ↑ Shiau, Carolyn; Toren, Andrew (2006). Toronto Notes 2006: Comprehensive Medical Reference (Review for MCCQE 1 and USMLE Step 2) (22nd edition (2006) ed.). Toronto Notes for Medical Students, Inc.. pp. Emerg.. ISBN 978-0968592861.
- ↑ URL: http://path.upmc.edu/cases/case139/micro.html. Accessed on: 6 January 2012.