Difference between revisions of "Invasive breast cancer"
| Line 494: | Line 494: | ||
===Microscopic=== | ===Microscopic=== | ||
Features: | Features: | ||
*Lesion confined to a | *Lesion confined to a cyst. | ||
**May have a thick fibrous capsule | **May have a thick fibrous capsule | ||
**The involved space | **The involved space is ''not'' lined by myoepithelial cells. | ||
*Loss of myoepithelial cells | *The cyst contains an abnormal epithelial proliferation with cribriform, solid or papillary architecture. | ||
*Scattered large cells with pale eosinophilic cytoplasm may be observed<ref>{{Cite journal | last1 = Collins | first1 = LC. | last2 = Schnitt | first2 = SJ. | title = Papillary lesions of the breast: selected diagnostic and management issues. | journal = Histopathology | volume = 52 | issue = 1 | pages = 20-9 | month = Jan | year = 2008 | doi = 10.1111/j.1365-2559.2007.02898.x | PMID = 18171414 }} | **Loss of myoepithelial cells within the epithelial proliferation is a '''key feature'''. | ||
**Scattered large cells with pale eosinophilic cytoplasm may be observed<ref>{{Cite journal | last1 = Collins | first1 = LC. | last2 = Schnitt | first2 = SJ. | title = Papillary lesions of the breast: selected diagnostic and management issues. | journal = Histopathology | volume = 52 | issue = 1 | pages = 20-9 | month = Jan | year = 2008 | doi = 10.1111/j.1365-2559.2007.02898.x | PMID = 18171414 }} | |||
</ref>. | </ref>. | ||
**These cells are so-called globoid cells or clear cells and are immunoreactive for GCDFP-15. | ***These cells are so-called globoid cells or clear cells and are immunoreactive for GCDFP-15. | ||
**They should not be mistaken for myoepithelial cells. | ***They should not be mistaken for myoepithelial cells. | ||
*Neoplastic epithelial cells: | **Neoplastic epithelial cells: | ||
**[[Nuclear atypia]] - including: nucleoli, [[nuclear pleomorphism]]. | ***[[Nuclear atypia]] - including: nucleoli, [[nuclear pleomorphism]]. | ||
** | |||
IHC: | |||
*Calponin/p63/SMA/CK5-6 | |||
**Loss of myoepithelial cells within the tumor. | |||
**Loss of myoepithelial cells at the cyst wall. | |||
*ER - Homogenous staining of the epithelial proliferation. | |||
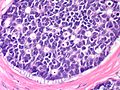
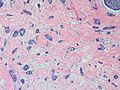
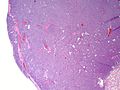
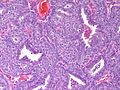
Photos: | Photos: | ||
Revision as of 09:24, 11 April 2015
The article deals with invasive breast cancer and the evaluation of hormone receptor & HER2 status. Non-invasive breast cancer is dealt with in non-invasive breast cancer.
Introduction
Overview of invasive breast cancer subtypes
Common epithelial subtypes
Type and percentage of breast carcinomas:[1]
- Ductal - AKA no special type (NST) - 79%.
- Lobular - 10%.
- Cribriform / tubular - 6%.
- Mucinous (colloid) - 2%.
- Medullary - 2%.
- Papillary - 1%.
- Metaplastic - <1%.
Common stromal types
- Malignant phyllodes tumour.
- Angiosarcoma - post-radiation ~ 10 years.[2]
Good prognosis subtypes
Three good prognosis subtypes:[3]
- Tubular carcinoma.
- Mucinous carcinoma.
- Papillary carcinoma.
Comprehensive list of invasive breast cancer subtypes
Epithelial
Counterparts of in situ lesions:
- Invasive ductal carinoma, not otherwise specified.
- Invasive lobular carcinoma.
- Invasive cribriform carcinoma.
- Invasive papillary carcinoma.
- Invasive micropapillary carcinoma.
Other epithelial tumours:
- Tubular carcinoma.
- Medullary carcinoma.
- Mucinous carinoma.
- Metaplastic carcinoma.
- Neuroendocrine tumour.
- Apocrine carcinoma.
- Lipid-rich carcinoma.
- Secretory carcinoma.
- Oncocytic carcinoma.
- Glycogen-rich clear cell carcinoma.
Epithelial tumours seen in the salivary gland:
Seen in the skin:
Clinically diagnosed:
- Inflammatory carcinoma.
In situ lesions:
Proliferative lesions:
Non-specific:
- Microinvasive carcinoma.
Papillary:
- Papilloma.
- Atypical papilloma.
- Intraductal papillary carcinoma.
Adenomas:
- Ductal adenoma.
- Tubular adenoma.
- Lactating adenoma.
- Apocrine adenoma.
- Pleomorphic adenoma.
Myoepithelial
- Myoepitheliosis.
- Adenomyoepithelial adenosis.
- Adenomyoepithelioma.
- Malignant adenomyoepithelioma.
Mesenchymal tumours
- See: Soft tissue lesions.
Fibroepithelial tumours
- Fibroadenoma.
- Phyllodes tumour.
- Periductal stromal sarcoma, low grade.
- Mammary hamartoma.
Nipple lesions
- Nipple adenoma.
- Syringomatous adenoma.
- Paget disease of the breast.
Other
Familial breast cancer
Breast IHC
Molecular classification of invasive carcinoma
A molecular classification:[4]
| Type | Percentage | IHC | Histology | Prognosis/clinical |
|---|---|---|---|---|
| Luminal A | ~45% | ER+ PR+ HER2- | well-differentiated | good, chemo resistant |
| Luminal B | 17% | ER+ PR+ HER2+ | high grade | poor, +/- chemo responsive |
| Normal breast-like | ~8% | ER+ PR+ (?) HER2- | well-differentiated | good |
| Basal-like | ~20% | ER- PR- HER2- | poorly differentiated | aggressive, may have good chemo response, classic for BRCA1 mutation |
| HER2 positive | ~10% | ER- PR- (?) HER2+ | poorly differentiated | poor |
The above is not applied clinically. A panel of immunostains (ER, PR, HER2, EGFR, CK5/6) can reproduce the molecular groupings; however, these groupings originate from gene expression profiling studies[5]
A newer classification outlines 10 subtypes based on molecular drivers identified by analysis of genomic and transcriptomic data from 2,000 breast tumors.[6]
Basal-like breast carcinoma
- Overview:[7]
- A category of breast carcinomas defined by gene expression profiling.
- Not used in clinical practice.
- Somewhere between 15-30% of breast carcinomas.
- Can be roughly be identified by immunohistochemistry - basal markers (CK14, p63, calponin, SMA).
- Not derived from myoepithelial cells, merely express a phenotype more in keeping with basal cells than ductal cells.
- Most triple negative (ER, PgR, Her-2); therefore cannot be treated with the usual therapeutic agents.
- There is an association in young women between basal-like breast cancer and BRCA1 mutation.
- Discussions of BRCA1 associated tumors, TNBC and BLBC are typically muddied by the overlap.
- Increased incidence in some populations - African-Americans, young women
- Sporadic basal-like cancers do not have a BRCA1 mutation but may have a dysfunctional BRCA1 pathway.
- p53 mutations are frequent.
- This molecular group includes a variety of morphologic phenotypes including:
- High grade invasive ductal carcinoma of no special type.
- Medullary-like carcinoma (a carcinoma with some but not all the features of medullary carcinoma).
- Medullary carcinoma
- Metaplastic carcinoma.
- Adenoid cystic carcinoma.
- Secretory carcinoma.
- Classic morphological clues of a basal type cancer usually refer to medullary carcinoma features:
- Relatively circumscribed.
- Geographic necrosis.
- Abundant mitoses.
- Pushing margins.
- Central fibrosis or necrosis.
- High histological grade.
- Exceptionally high mitotic rate.
- Pushing borders.
- Conspicuous lymphocytic infiltrate.
- Behaviour:
- Basal-like breast cancer is a heterogeneous group.
- The behaviour of basal-like breast cancer appears to fall into two groups:
- The tumours that are by nature low grade (ie adenoid cystic carcinoma) and/or do not metastasise have a better prognosis than other types of breast carcinoma.
- The tumours with early metastasis that may behave more aggressively
- Hematogenous spread -greater tendency to metastasise to visceral sites (notably lung and brain) instead of to nodes and bone.
- Many have a complete response to chemotherapy and survival rates similar to typical breast cancer
- Non-complete response to chemotherapy is associated with low survival at 5 years.
Other sources Minireview: Basal-Like Breast Cancer: From Molecular Profiles to Targeted Therapies <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3035993/>
Triple Negative Breast Carcinoma
Features:[8]
- A category of breast carcinomas defined by immunohistochemical/FISH expression of ER, PR and HER2.
- Important to identify in clinical practice.
- About 15% of breast carcinomas.
- Important group due to a lack of tailored therapies for this group
- Some triple negatives also express androgen receptor and have and [apocrine carcinoma] morphology.[9]
- May respond to therapies targeting the androgen receptor.
- BCL11A overexpression recently identified as an oncogenic driver for some triple negatives [10]
- Targeted therapies may include inhibitors of BCL11A.
- Some triple negatives also express androgen receptor and have and [apocrine carcinoma] morphology.[9]
- Triple-negative and basal-like phenotypes are not synonymous but overlap
- About 70% of triple-negative tumours are basal-like.
- About 70% of basal-like tumors are triple-negative tumours.
- Discussions of BRCA1 associated tumors, TNBC and BLBC are typically muddied by the overlap.
- Classic 'morphological clues' to a triple negative cancer usually refer to medullary carcinoma features.
Immunostains for typing and diagnosis
DCIS versus LCIS
Tabular comparison for DCIS versus LCIS:[11][12]
| Disease | E-cadherin | Beta-catenin | 34betaE12 | CAM5.2 (CK8) |
|---|---|---|---|---|
| DCIS | +ve | +ve | -ve | +ve peripheral cytoplasm |
| LCIS | -ve | -ve | +ve perinuclear | +ve perinuclear |
Invasive versus non-invasive
Myoepithelial markers - typically lost in invasive carcinoma:[13]
| Stain | Location | Notes |
|---|---|---|
| p63 | nuclear | up to 10% of invasive tumours +ve[14] |
| Smooth muscle actin (SMA) | cytoplasmic | stains myofibroblasts & blood vessels |
| Calponin | cytoplasmic | stains myofibroblasts & blood vessels |
| Smooth muscle myosin heavy chain (SMM-HC) |
cytoplasmic | stains myofibroblasts & blood vessels |
Usual ductal hyperplasia versus ductal carcinoma in situ
Markers for UDH versus DCIS:[14]
| Disease | CK5/6 | ER |
|---|---|---|
| UDH | diffuse +ve | patchy +ve |
| DCIS | -ve | diffuse +ve |
Lymphovascular invasion
- D2-40 - marks the lymphatic spaces.[15][16]
- CD31 - marks lymphovascular spaces.
- CD34 - marks lymphovascular spaces, less specific than CD31.
- Immunostaining of any sentinel lymph nodes - to look for isolated tumour cells and small lymph node mets.
- Sunnybrook uses CAM5.2.
- ER (estrogen receptor).
- Positive in most breast cancers; +ve in ~75-80%.[17]
- PR (progesterone receptor).
- Positive in most breast cancers; +ve in ~65-70%.[17]
- HER2/neu (HER2).
Note:
- Male breast cancer is usually hormone receptor positive (~97%), and HER2 positivity is quite rare (~6%).[20]
ER & PR scoring
Nuclear staining:[17]
- Give a percentage, i.e. 0-100%.
- Important cut points: 1% and 10%.
- 0% = negative - not treated.
- <10% = low positivity - treated.
- Important cut points: 1% and 10%.
Notes:
- Normal breast epithelial cells have a patchy staining for ER and PR.
- Evaluated on the invasive component.
HER2 scoring
Immunohistochemical based testing:[21][22]
| Score | Staining intensity | Cells stained (%) | Membrane staining | Management | Percentage of cases |
|---|---|---|---|---|---|
| 0 | no staining/barely visible | ≤10% | incomplete | No HER2 blocker | ~60% |
| 1+ | minimal/barely visible | >10% | incomplete | No HER2 blocker | ~10% |
| 2+ | weak-to-moderate | >10% | incomplete (circumferential) | Needs SISH or FISH | ~10% † |
| 2+ | intense | ≤10% | complete | Needs SISH or FISH | ~10% † |
| 3+ | intense staining | >10% ‡ | complete | HER2 blocker | ~20% |
Note for IHC:
- Normal breast epithelial cells do not stain with HER2.
- Evaluated on the invasive component.
- SISH = silver in situ hybridization.
- FISH = fluorescence in situ hybridization.
- † Together approximately 10%.
- ‡ The cut point was 10%, changed to 30% and then changed back to 10%.[21]
ISH based testing:[23]
| Result | Ratio criteria | Gene copy number criteria |
|---|---|---|
| Positive | ≥2.0 HER2/CEP17 | ≥6.0 copies of HER2/cell |
| Equivocal | <2.0 HER2/CEP17 (required) | 4.0-6.0 copies of HER2/cell |
| Negative | <2.0 HER2/CEP17 | <4.0 copies of HER2/cell |
Note for ISH:
- Can be called positive based on either ratio criteria or gene copy number criteria.
Clinical
- ER & PR status determine whether a patient will get tamoxifen or other estrogen receptor modulators, such as raloxifene (Evista).
- HER2 status determines whether patient will get traztuzumab (Herceptin) or other HER2/neu modulators.
Characteristics of the subtypes
Invasive ductal carcinoma of the breast
Invasive lobular carcinoma
- Abbreviated ILC.
- AKA lobular carcinoma.
Medullary breast carcinoma
- AKA medullary carcinoma of the breast.
Tubular carcinoma of the breast
- AKA tubular carcinoma.
Metaplastic breast carcinoma
- AKA metaplastic carcinoma.
Invasive micropapillary carcinoma of the breast
- AKA micropapillary carcinoma.
General
- Poor prognosis.
- LVI common.[24]
Microscopic
Features:
- Clear spaces/clefting around (small) nests of tumour - diffuse/through-out the tumour - key feature.
- Described as "small clusters of tumour lying within dilated vascular channel-like spaces".[25]
Note:
- Ductal carcinoma commonly has clefting... but it isn't diffuse.
Images:
- Invasive micropapillary carcinoma (flickr.com/euthman).
- Invasive micropapillary carcinoma - crappy image (breast-cancer.ca).[26]
IHC
- EMA +ve (periphery of nests); described as inside-out pattern.[25]
- E-cadherin +ve (centre of nests). (???)
- p63 +ve/-ve.
Apocrine carcinoma of the breast
Mucinous breast carcinoma
Adenoid cystic carcinoma of the breast
- AKA breast adenoid cystic carcinoma.
General
- Like tumour of the salivary gland.
- Very rare <0.1% of breast malignancies.[27]
- Good prognosis.[27]
Microscopic
- See: Adenoid cystic carcinoma article.
IHC
- One of the 'good prognosis' triple negative breast cancers - ER, PR and HER2 negative[28]
- Basal-like breast cancer - p63 and CK5/6 positive[29]
CD117 positive[30]
DDx:
- Cribriform DCIS.
- Invasive cribriform carcinoma.
- Collagenous spherulosis.
Images
Other Resources
Pathology Outlines [1]
Intracystic papillary carcinoma of the breast
- AKA encapsulated papillary carcinoma of the breast, abbreviated EPC.
General
- Very good prognosis[31] - it is similar to DCIS.
- Classical menopausal women.
- ~30% present with bloody discharge.[32]
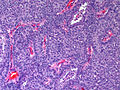
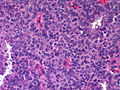
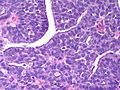
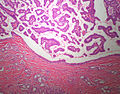
Microscopic
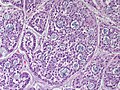
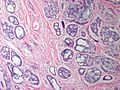
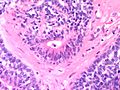
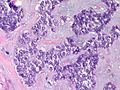
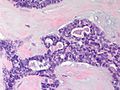
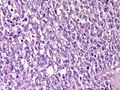
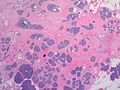
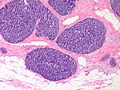
Features:
- Lesion confined to a cyst.
- May have a thick fibrous capsule
- The involved space is not lined by myoepithelial cells.
- The cyst contains an abnormal epithelial proliferation with cribriform, solid or papillary architecture.
- Loss of myoepithelial cells within the epithelial proliferation is a key feature.
- Scattered large cells with pale eosinophilic cytoplasm may be observed[33].
- These cells are so-called globoid cells or clear cells and are immunoreactive for GCDFP-15.
- They should not be mistaken for myoepithelial cells.
- Neoplastic epithelial cells:
- Nuclear atypia - including: nucleoli, nuclear pleomorphism.
IHC:
- Calponin/p63/SMA/CK5-6
- Loss of myoepithelial cells within the tumor.
- Loss of myoepithelial cells at the cyst wall.
- ER - Homogenous staining of the epithelial proliferation.
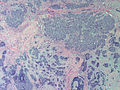
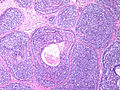
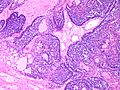
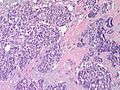
Photos:
WebPathology<http://www.webpathology.com/images/noimage.gif> WebPathology<http://www.webpathology.com/images/noimage.gif> WebPathology<http://www.webpathology.com/images/noimage.gif>
- Intraductal papilloma.
- Absent or scant stroma favors papillary carcinoma over papilloma.
- Is there a single cell or dual cell population in the lesion?
- ER staining will be heterologous in a benign lesion.
- Myoepithelial markers (calponin/p63/SMA +ve)s hould be positive in a benign lesion.
- Papillary ductal carcinoma in situ
- Papillary DCIS shows myoepithelial cells (calponin/p63/SMA +ve) at the periphery of the involved spaces
- But papillary DCIS should be negative for myoepithelial cells within the focus of DCIS
- Papillary intracystic carcinoma does not show myoepithelial cells at the periphery of the involved spaces
- Invasive carcinoma arising in association with papillary intracystic carcinoma
- Epithelial entrapment in the encysting fibrous tissue should not be interpreted as invasion.
- Carcinoma must be seen in the breast tissue outside the encysting fibrous tissue.
- Adenoid cystic carcinoma of the breast
- The solid variant looks basaloid - solid adenoid cystic carcinoma or a 'basal-like' carcinoma should be considered in these cases.
Notes
- Adequately and carefully sample the specimen to exclude an invasive component.
- Report only the size of the invasive component (if present) to prevent over-estimation of tumor stage.
IHC
- Loss of myoepithelial markers within the lesion.
Glycogen-rich clear cell carcinoma of the breast
- Abbreviated GRCC.
Secretory carcinoma of the breast
- AKA secretory breast carcinoma, abbreviated SBC.
Grading breast cancer
Most common system: Nottingham (aka Scarff-Bloom-Richardson) which is based on:
- Nuclear grade.
- Small, regular (1.5-2x RBC dia.) = 1.
- Moderated variability = 2.
- Marked variation (>2.5x RBC dia.) = 3.
- Tubule formation.
- Majority of tumour - tubules >75% = 1.
- Moderate - 10% to 75% = 2.
- Minimal <10% = 3.
- Mitotic rate.
- 0-5 mitosis/10 HPF (1.52 mm^2 --or-- 0.0152 mm^2 * 10) = 1.
- 6-10 mitosis/10 HPF (1.52 mm^2) = 2.
- >11 mitosis/10 HPF (1.52 mm^2) = 3.
Mnemonic: TMN = tubule formation, mitotic rate, nuclear grade.
Notes:
- Elston & Ellis devised the system that is used.[36] They also wrote a follow-up article in 2002.[37]
Note about mitosis counting
- One MUST adjust for the size of the field of view.
- Most of the Resident scopes have an eye piece diameter of 22 mm. Therefore, the field diameter at 40 X is approximately 22 mm / 40 X ~= 0.55 mm and the field of view is pi/4*(0.55 mm)^2 = 0.2376 mm^2.
- Thus, on a resident scope (with a FOV of 0.2376 mm^2) one should sample 6 or 7 fields of view (FsOV).
- Calculation: 1.52 mm^2 (sampling area) / 0.2376 mm^2 (area / FOV ) = 6.40 FsOV.
- Thus, on a resident scope (with a FOV of 0.2376 mm^2) one should sample 6 or 7 fields of view (FsOV).
- RANT: Sampling 10 fields, where the field of view (FOV) is 0.152 mm^2, is not the same as sampling ten fields, where the FOV is 0.312 mm^2. It surprises me that Elston & Ellis ignore the fact that "10 HPFs" on different microscopes represent different sample areas and that they do not standardize the sampling area.
Calculating Nottingham score
- Grade I = 3-5 points.
- Grade II = 6-7 points.
- Grade III = 8-9 points.
Notes:
- I've found most tumours are grade II.
- The mitotic score is usually 1/3.
- The nuclear score is rarely 1/3 -- even in the tubular subtype.[38]
Staging breast cancer
Sentinel lymph node sampling in breast cancer
General
- Selective sampling of lymph nodes.
- Used for staging.
- Positive LNs = poorer prognosis.
Notes:
- If there is no palpable disease, there is no mortality benefit from axillary lymph node dissection, i.e. positive axillary lymph nodes can be left in situ without affecting outcome.[39]
- This does not negate the fact that a positive sentinel LN biopsy (vs. negative sentinel LN biopsy) portends a poorer prognosis.
Microscopic
Features:
- Atypical cells.
- Nuclear changes of malignancy:
- Nuclear enlargement + variation in size.
- Variation in shape.
- Hyperchromasia and variation in staining.
- Usually in the subcapsular sinuses.
- Nuclear changes of malignancy:
Pitfalls:
- Naevus cell rests.[40]
IHC
Some hospitals use:
- CAM5.2 (LMWK) - to look for isolated tumour cells and small lymph node metstases.
N stage
Sampling usually selective, i.e. sentinel lymph nodes only.
Indictionas for lymph node sampling
Indications for lymph node sampling:[41]
- Extensive DCIS.
- Biopsy suspicious for invasion or with microinvasion.
- Clinical findings (large palable mass) or radiology findings (irregular features) suggestive of invasion.
- Planned mastectomy.
Definitions
Definitions:[42]
- Isolated tumour cells: <=0.2 mm or <=200 cells -- in a single cross-section. †
- Micrometastasis: <=0.2 cm and ( >0.2 mm or >200 cells ).
- Macrometastasis: >0.2 cm.
Notes:
- † The American Cancer Society web site says "or".[42] The CAP protocol says "and/or" and notes it is all subjective.
- Isolated tumour cells are essentially ignored if the there is at least one macrometastasis.
Details
Lymph nodes:[43]
- pN0: nil.
- pN0(i+): <=0.2 mm and <200 cells.
- pN1: 1-3 axillary LNs or internal mammary LNs.
- pN1mi: <=0.2 cm and ( >0.2 mm or >=200 cells ).
- pN1a.
- pN1b.
- PN1c.
- pN2 4-9 positive LNs; internal mammary LNs or axillary LNs.
- pN3.
T stage
- pT1: <= 20 mm.
- pT1mic <= 1 mm.
- pT1a > 1 mm and <= 5 mm.
- pT1b > 5 mm and <= 10 mm.
- pT1c > 10 mm and <= 20 mm.
- pT2: > 20 mm and <= 50 mm
- pT3: > 50 mm.
- pT4: chest wall or skin involvement.
Notes:
- Values should be rounded to the nearest millimetre.
- Therefore:
- 1.4 mm would be pT1mic.
- 1.5 mm would be pT1a.
- Therefore:
M stage
Distant metastasis:
- cM0(i+) <=0.2 mm focus of tumour cells, without clinical signs and symptoms.
- pM1 focus of tumour cells > 0.2 mm.
Lymphovascular invasion
In the context of breast pathology, the Rosen criteria for LVI are widely excepted, and are as follows:[46][47]
- Must be outside of the tumour proper.
- LVI is usually very close -- typically within 0.1 cm.
- Contour of cells should differ from possible vessel wall.
- DCIS with retraction artifact mimicing LVI has a contour that matches its surrounding fibrous tissue.
- Endothelium (usu. flat) should be visible.
- Lymphatics are found adjacent to blood vessels - vessels should be present in the vicinity.
Memory device LUBE-O:
- LVI has a Unique contour, Blood vessels and Endothelium in the vicinity, and is Outside of the tumour.
Note:
- LVI does not affect the stage.
Other
Paget's disease
General
- Associated with underlying breast carcinoma.[48]
Notes:
- Unrelated to Paget disease of the bone.
Microscopic
Features:[48]
- Cells in the epidermis:
- Epitheliod morphology (round/ovoid).
- Cells nested or single.
- Clear/pale cytoplasm key feature - may also be eosinophilic.
- Large nucleoli.
Images:
IHC & DDx:
- See Paget disease.
Trivia
Tumour size and lymph node metastases
There is a paper[49] that calculates the probability of lymph node mets based on tumour size. The developed formula is:
Where:
- = the probability of the lymph nodes being positive.
- D = the largest dimension of the tumour in millimetres.
- Z = 1.0041.
- = 0.019.
Selected values
| Tumour size (mm) | Probability |
| 5 | 9 % |
| 10 | 17 % |
| 15 | 25 % |
| 20 | 32 % |
| 25 | 38 % |
| 30 | 44 % |
| 35 | 49 % |
| 40 | 54 % |
| 45 | 58 % |
| 50 | 62 % |
Natural history
There is a theory that up to 22% of small (radiographically detected) breast tumours regress, based on an analysis in a large population.[50] The study is supported by NCI's SEER data.[51] Also, it generated many comments.[50]
Missed macrometastases
The effect of missed macrometastases is small; this implies using IHC to look for isolated tumour cells is money that isn't well spent.[52]
See also
References
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1143. ISBN 0-7216-0187-1.
- ↑ URL: http://www.breastpathology.info/Case_of_the_month/2007/COTM_1007%20discussion.html. Accessed on: 28 November 2010.
- ↑ URL: http://emedicine.medscape.com/article/1947145-overview. Accessed on: 24 August 2012.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 547. ISBN 978-1416054542.
- ↑ Tang, P.; Skinner, KA.; Hicks, DG. (Sep 2009). "Molecular classification of breast carcinomas by immunohistochemical analysis: are we ready?". Diagn Mol Pathol 18 (3): 125-32. doi:10.1097/PDM.0b013e31818d107b. PMID 19704256.
- ↑ Curtis, C.; Shah, SP.; Chin, SF.; Turashvili, G.; Rueda, OM.; Dunning, MJ.; Speed, D.; Lynch, AG. et al. (Jun 2012). "The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups.". Nature 486 (7403): 346-52. doi:10.1038/nature10983. PMID 22522925.
- ↑ Badve, S.; Dabbs, DJ.; Schnitt, SJ.; Baehner, FL.; Decker, T.; Eusebi, V.; Fox, SB.; Ichihara, S. et al. (Feb 2011). "Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists.". Mod Pathol 24 (2): 157-67. doi:10.1038/modpathol.2010.200. PMID 21076464.
- ↑ Badve, S.; Dabbs, DJ.; Schnitt, SJ.; Baehner, FL.; Decker, T.; Eusebi, V.; Fox, SB.; Ichihara, S. et al. (Feb 2011). "Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists.". Mod Pathol 24 (2): 157-67. doi:10.1038/modpathol.2010.200. PMID 21076464.
- ↑ Niemeier, LA.; Dabbs, DJ.; Beriwal, S.; Striebel, JM.; Bhargava, R. (Feb 2010). "Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation.". Mod Pathol 23 (2): 205-12. doi:10.1038/modpathol.2009.159. PMID 19898421.
- ↑ Khaled, WT.; Choon Lee, S.; Stingl, J.; Chen, X.; Raza Ali, H.; Rueda, OM.; Hadi, F.; Wang, J. et al. (2015). "BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells.". Nat Commun 6: 5987. doi:10.1038/ncomms6987. PMID 25574598.
- ↑ O'Malley, Frances P.; Pinder, Sarah E. (2006). Breast Pathology: A Volume in Foundations in Diagnostic Pathology series (1st ed.). Churchill Livingstone. pp. 275. ISBN 978-0443066801.
- ↑ Yeh IT, Mies C (March 2008). "Application of immunohistochemistry to breast lesions". Arch. Pathol. Lab. Med. 132 (3): 349-58. PMID 18318578. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=132&page=349.
- ↑ Lester, Susan Carole (2010). Manual of Surgical Pathology (3rd ed.). Saunders. pp. 88. ISBN 978-0-323-06516-0.
- ↑ 14.0 14.1 O'Malley, Frances P.; Pinder, Sarah E. (2006). Breast Pathology: A Volume in Foundations in Diagnostic Pathology series (1st ed.). Churchill Livingstone. pp. 276. ISBN 978-0443066801.
- ↑ Ordóñez NG (March 2006). "Podoplanin: a novel diagnostic immunohistochemical marker". Adv Anat Pathol 13 (2): 83-8. doi:10.1097/01.pap.0000213007.48479.94. PMID 16670463.
- ↑ Kahn HJ, Marks A (September 2002). "A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors". Lab. Invest. 82 (9): 1255-7. PMID 12218087.
- ↑ 17.0 17.1 17.2 17.3 Lester, Susan Carole (2005). Manual of Surgical Pathology (2nd ed.). Saunders. pp. 241-2. ISBN 978-0443066450.
- ↑ Gallardo, A.; Lerma, E.; Escuin, D.; Tibau, A.; Muñoz, J.; Ojeda, B.; Barnadas, A.; Adrover, E. et al. (Apr 2012). "Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas.". Br J Cancer 106 (8): 1367-73. doi:10.1038/bjc.2012.85. PMID 22454081.
- ↑ Jensen, JD.; Knoop, A.; Laenkholm, AV.; Grauslund, M.; Jensen, MB.; Santoni-Rugiu, E.; Andersson, M.; Ewertz, M. (Dec 2011). "PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome in HER2-positive early-stage breast cancer patients treated with adjuvant chemotherapy and trastuzumab.". Ann Oncol. doi:10.1093/annonc/mdr546. PMID 22172323.
- ↑ Schildhaus, HU.; Schroeder, L.; Merkelbach-Bruse, S.; Binot, E.; Büttner, R.; Kuhn, W.; Rudlowski, C. (Sep 2013). "Therapeutic strategies in male breast cancer: Clinical implications of chromosome 17 gene alterations and molecular subtypes.". Breast. doi:10.1016/j.breast.2013.08.008. PMID 24080492.
- ↑ 21.0 21.1 Rakha, EA.; Starczynski, J.; Lee, AH.; Ellis, IO. (Apr 2014). "The updated ASCO/CAP guideline recommendations for HER2 testing in the management of invasive breast cancer: a critical review of their implications for routine practice.". Histopathology 64 (5): 609-15. doi:10.1111/his.12357. PMID 24382093.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/breast_biomarker_template.pdf. Accessed on: October 7, 2014.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/breast_biomarker_template.pdf. Accessed on: October 7, 2014.
- ↑ Yu, JI.; Choi, DH.; Park, W.; Huh, SJ.; Cho, EY.; Lim, YH.; Ahn, JS.; Yang, JH. et al. (Jun 2010). "Differences in prognostic factors and patterns of failure between invasive micropapillary carcinoma and invasive ductal carcinoma of the breast: matched case-control study.". Breast 19 (3): 231-7. doi:10.1016/j.breast.2010.01.020. PMID 20304650.
- ↑ 25.0 25.1 Yamaguchi, R.; Tanaka, M.; Kondo, K.; Yokoyama, T.; Kaneko, Y.; Yamaguchi, M.; Ogata, Y.; Nakashima, O. et al. (Aug 2010). "Characteristic morphology of invasive micropapillary carcinoma of the breast: an immunohistochemical analysis.". Jpn J Clin Oncol 40 (8): 781-7. doi:10.1093/jjco/hyq056. PMID 20444748.
- ↑ URL: http://www.breast-cancer.ca/type/micropapillary-breast-carcinoma.htm. Accessed on: 30 May 2012.
- ↑ 27.0 27.1 Boujelbene, N.; Khabir, A.; Boujelbene, N.; Jeanneret Sozzi, W.; Mirimanoff, RO.; Khanfir, K. (Dec 2011). "Clinical review - Breast adenoid cystic carcinoma.". Breast. doi:10.1016/j.breast.2011.11.006. PMID 22154460.
- ↑ Azoulay, S.; Laé, M.; Fréneaux, P.; Merle, S.; Al Ghuzlan, A.; Chnecker, C.; Rosty, C.; Klijanienko, J. et al. (Dec 2005). "KIT is highly expressed in adenoid cystic carcinoma of the breast, a basal-like carcinoma associated with a favorable outcome.". Mod Pathol 18 (12): 1623-31. doi:10.1038/modpathol.3800483. PMID 16258515.
- ↑ Azoulay, S.; Laé, M.; Fréneaux, P.; Merle, S.; Al Ghuzlan, A.; Chnecker, C.; Rosty, C.; Klijanienko, J. et al. (Dec 2005). "KIT is highly expressed in adenoid cystic carcinoma of the breast, a basal-like carcinoma associated with a favorable outcome.". Mod Pathol 18 (12): 1623-31. doi:10.1038/modpathol.3800483. PMID 16258515.
- ↑ Azoulay, S.; Laé, M.; Fréneaux, P.; Merle, S.; Al Ghuzlan, A.; Chnecker, C.; Rosty, C.; Klijanienko, J. et al. (Dec 2005). "KIT is highly expressed in adenoid cystic carcinoma of the breast, a basal-like carcinoma associated with a favorable outcome.". Mod Pathol 18 (12): 1623-31. doi:10.1038/modpathol.3800483. PMID 16258515.
- ↑ Rakha, EA.; Gandhi, N.; Climent, F.; van Deurzen, CH.; Haider, SA.; Dunk, L.; Lee, AH.; Macmillan, D. et al. (Aug 2011). "Encapsulated papillary carcinoma of the breast: an invasive tumor with excellent prognosis.". Am J Surg Pathol 35 (8): 1093-103. doi:10.1097/PAS.0b013e31821b3f65. PMID 21753694.
- ↑ Rodríguez, MC.; Secades, AL.; Angulo, JM. (Nov 2010). "Best cases from the AFIP: intracystic papillary carcinoma of the breast.". Radiographics 30 (7): 2021-7. doi:10.1148/rg.307105003. PMID 21057133.
- ↑ Collins, LC.; Schnitt, SJ. (Jan 2008). "Papillary lesions of the breast: selected diagnostic and management issues.". Histopathology 52 (1): 20-9. doi:10.1111/j.1365-2559.2007.02898.x. PMID 18171414.
- ↑ Collins, LC.; Schnitt, SJ. (Jan 2008). "Papillary lesions of the breast: selected diagnostic and management issues.". Histopathology 52 (1): 20-9. doi:10.1111/j.1365-2559.2007.02898.x. PMID 18171414.
- ↑ Pathmanathan, N.; Albertini, AF.; Provan, PJ.; Milliken, JS.; Salisbury, EL.; Bilous, AM.; Byth, K.; Balleine, RL. (Jul 2010). "Diagnostic evaluation of papillary lesions of the breast on core biopsy.". Mod Pathol 23 (7): 1021-8. doi:10.1038/modpathol.2010.81. PMID 20473278.
- ↑ Elston CW, Ellis IO (September 2002). "Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991; 19; 403-410". Histopathology 41 (3A): 151–2, discussion 152–3. PMID 12405945.
- ↑ Elston CW, Ellis IO (November 1991). "Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up". Histopathology 19 (5): 403–10. PMID 1757079.
- ↑ MUA. 20 January 2009.
- ↑ Giuliano AE, Hunt KK, Ballman KV, et al. (February 2011). "Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial". JAMA 305 (6): 569–75. doi:10.1001/jama.2011.90. PMID 21304082.
- ↑ URL: http://www.breastpathology.info/Case_of_the_month/2007/COTM_1107%20discussion.html. Accessed on: 28 November 2010.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2009/InvasiveBreast_09protocol.pdf. Accessed on: 2 April 2012.
- ↑ 42.0 42.1 URL: http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-staging. Accessed on: 8 July 2010.
- ↑ URL: http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-staging. Accessed on: 8 July 2010.
- ↑ URL: http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-staging. Accessed on: 8 July 2010.
- ↑ URL: http://www.cancerhelp.org.uk/type/breast-cancer/treatment/tnm-breast-cancer-staging. Accessed on: 9 July 2010.
- ↑ Rosen, PP. (1983). "Tumor emboli in intramammary lymphatics in breast carcinoma: pathologic criteria for diagnosis and clinical significance.". Pathol Annu 18 Pt 2: 215-32. PMID 6674861.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2009/InvasiveBreast_09protocol.pdf. Accessed on: 5 August 2011.
- ↑ 48.0 48.1 URL: http://emedicine.medscape.com/article/1101235-diagnosis
- ↑ Porembka, MR.; Abraham, RL.; Sefko, JA.; Deshpande, AD.; Jeffe, DB.; Margenthaler, JA. (Oct 2008). "Factors associated with lymph node assessment in ductal carcinoma in situ: analysis of 1988-2002 seer data.". Ann Surg Oncol 15 (10): 2709-19. doi:10.1245/s10434-008-9947-5. PMID 18483831. http://onlinelibrary.wiley.com/doi/10.1002/cncr.24592/pdf.
- ↑ 50.0 50.1 Zahl, PH.; Maehlen, J.; Welch, HG. (Nov 2008). "The natural history of invasive breast cancers detected by screening mammography.". Arch Intern Med 168 (21): 2311-6. doi:10.1001/archinte.168.21.2311. PMID 19029493.
- ↑ Jatoi, I.; Anderson, WF. (May 2009). "Breast cancer overdiagnosis with screening mammography.". Arch Intern Med 169 (10): 999-1000, author reply 1000-1. doi:10.1001/archinternmed.2009.95. PMID 19468099.
- ↑ Weaver, DL.; Ashikaga, T.; Krag, DN.; Skelly, JM.; Anderson, SJ.; Harlow, SP.; Julian, TB.; Mamounas, EP. et al. (Feb 2011). "Effect of occult metastases on survival in node-negative breast cancer.". N Engl J Med 364 (5): 412-21. doi:10.1056/NEJMoa1008108. PMID 21247310.