Difference between revisions of "Stomach"
(→Sleeve gastrectomy: split out) |
|||
| Line 146: | Line 146: | ||
====Sleeve gastrectomy==== | ====Sleeve gastrectomy==== | ||
{{Main|Sleeve gastrectomy}} | |||
=Introduction= | =Introduction= | ||
Revision as of 14:16, 2 May 2018
Stomach is an important organ for pathologists. It is often inflamed and may be a site that cancer arises from. Gastroenterologists often biopsy the organ. Surgeons take-out the organ. It connects the esophagus to the duodenum. An introduction to gastrointestinal pathology is in the gastrointestinal pathology article.
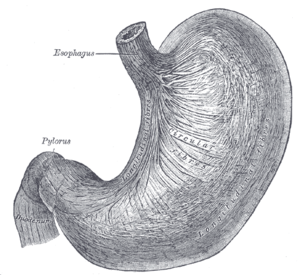
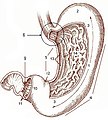
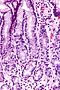
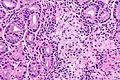
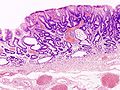
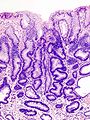
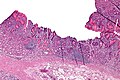
Normal stomach
Gross anatomy
- Cardia - first part of the stomach; joins with esophagus.
- Fundus - superior portion - not attached directly to the esophagus.
- Body - contains parietal cells.
- Pylorus - distal (think pyloric stenosis); it joins with the duodenum.
- AKA antrum.
Image
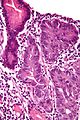
Microscopic
Foveolar cells versus intestinal goblet cells
- Intestinal goblet cells - clear mucin.
- Foveolar cells - eosinophilic contents.
Stomach versus intestine
A tabular comparison:[1]
| Feature | Intestine | Stomach |
|---|---|---|
| Spacing | Goblets cell - spaced | Foveolar cells - beside one another |
| Morphology of epithelial cells | columnar | tall columnar (Champagne flute) |
| Vesicle at luminal surface | touching/small opening | wide open |
| PAS-D | -ve (???) | +ve[2] |
| Villin stain[3][4] | +ve | -ve |
| Images | Tubular adenoma - goblet cells on right of image (WC) |
Gastric biopsy (microscopy-uk.org.uk), Stomach with cancer - PAS (WC), Stomach (WC) |
Notes:
- Intraepithelial lymphocytes in the gastric mucosa have a clear halo around 'em.[5]
- Memory device: Folveolar cells have friends, i.e. they are close to other foveolar cells.
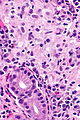
Gastric antrum versus gastric body
| Cell | Body | Antrum | Histology | Image |
|---|---|---|---|---|
| Parietal cell | abundant | few or none | parietal cells: intensely eosinophilic cytoplasm |
|
| Chief cell | present | absent | chief cells: basophilic cytoplasm, IHC: +ve for pepsinogen I |
|
| G cell | absent | present | fried egg appearance (clear cytoplasm, round nucleus); look at high power - usu. middle 1/3 of gland,[6] IHC: +ve for gastrin. |
|
| Surface | flat | blunted villi | antrum is somewhat duodenum-like |
|
| Gastric glands / mucosa |
thick | thin | not so useful for discrimination |
body - thick, body & antrum |
Notes:
- G cells may superficially resemble intraepithelial lymphocytes.
- G cell nucleus is usu. perfectly round and slightly larger (diameter of 12 micrometers?) than a lymphocyte nucleus (diameter ~ 9-10 micrometers?).
Sign out
Short version
Stomach, Biopsy: - Antral-type gastric mucosa within normal limits.
Stomach, Biopsy: - Body and antral-type gastric mucosa within normal limits.
Stomach, Biopsy: - Antral-type gastric mucosa within normal limits. - NEGATIVE for Helicobacter-like organisms.
Block letters
STOMACH, BIOPSY: - BODY AND ANTRAL-TYPE GASTRIC MUCOSA WITHIN NORMAL LIMITS.
STOMACH, BIOPSY: - BODY AND ANTRAL-TYPE GASTRIC MUCOSA WITHIN NORMAL LIMITS. - NEGATIVE FOR HELICOBACTER-LIKE ORGANISMS.
STOMACH, BIOPSY: - ANTRAL-TYPE GASTRIC MUCOSA WITHIN NORMAL LIMITS. - NEGATIVE FOR HELICOBACTER-LIKE ORGANISMS.
Long version
STOMACH, BIOPSY: - BODY/ANTRAL-TYPE GASTRIC MUCOSA. - INFLAMMATION: ABSENT. - ATROPHY: ABSENT. - INTESTINAL METAPLASIA: ABSENT. - HELICOBACTER-LIKE ORGANISMS: NOT IDENTIFIED WITH ROUTINE STAINS. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY.
Sleeve gastrectomy
Introduction
Useful stains for stomach
- Cresyl violet stain[7] - used to find H. pylori.[8]
- Alcian blue stain - used to find mucin[9] which is present in intestinal metaplasia
Things to look for...
- Parietal cells (indicate you're in the body of the stomach) - pink (eosinophilic) cytoplasm.
- Lack of parietal cells -- DDx: Bx of antrum (pylorus), Bx of cardia, pernicious anemia.
- Goblet cells = intestinal metaplasia.
- Architectural distortion of gastric glands - suspect cancer.
- Signet ring cells = (usually) gastric carcinoma.
- Can be very easy to miss in some biopsies.
- Inflammation + small bacteria = suspect H. pylori gastritis.
Some patterns
Gastric atrophy
General
- Has a wide differential diagnosis.
Microscopic
Can take three general forms:
- Intestinal metaplasia - see intestinal metaplasia section.
- Pseudopyloric metaplasia; gastric body looks like gastric antrum.
- Characterized by foveolar hyperplasia.
- Cell loss without replacement.
- Clue is deep inflammation in the body.
Plasma cells in the stomach
DDx of plasmacytosis:
- Plasma cell neoplasm.
- Syphilis.
- Chronic gastritis.
Granulomatous gastritis
- Usual DDx of granulomatous disease (see Basics article):
- DNF AAII:
- Drugs, Neoplasms, Foreign body, Autoimmune, Allergic, Infectious, Idiopathic.
- DNF AAII:
Important ones:
- Autoimmune - Crohn's disease.
- Infectious - Tuberculosis.
- Idiopathic - Sarcoidosis.
Non-neoplastic disease
Peptic ulcer disease
- Abbreviated PUD.
- For duodenal manifestations see Peptic duodenitis.
General
- Benign.
Complications:
- Hemorrhage.
- Obstruction.
- Perforation - can be fatal.
Etiology - typically:[11]
Gross
Features:
- Typically in the duodenum; duodenum:stomach = ~4:1.
- Epithelial defect with punched-out edges (suggestive of a benign process).
Note:
- Heaped edges - suggestive of cancer.
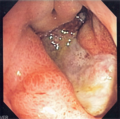
Endoscopic image
Microscopic
Features:
- Loss of epithelium.
- Inflammation.
- +/-Helicobacter organisms - see Helicobacter gastritis.
Gastritis
Helicobacter gastritis
Intestinal metaplasia of the stomach
Inflammatory bowel disease and the stomach
- Histopathologic findings are usually non-specific.
- Conventional thinking was upper GI involvement = Crohn's disease; this is changing.[12]
Endoscopic/gross
Features - Crohn's:[13]
- +/-Linear fissures, erosions, ulcers, cobblestoning.
- May mimic linitis plastica.
Microscopic
Features:[14]
- Focal inflammation.
- Common finding - non-specific.
- +/-Granulomas.
Note:
- Granulomas in Crohn's gastritis present 7-34% of the time.[13]
Images
Miscellaneous
This is a grab bag of stuff seen in the stomach. Some of it is quite rare.
Gastric antral vascular ectasia
Reactive gastropathy
Autoimmune metaplastic atrophic gastritis
- AKA autoimmune gastritis.
Collagenous gastritis
Gastritis cystitis profunda
- AKA Gastritic cystica profunda.[citation needed]
General
- May be associated with glandular proliferation as well.[15] (???)
- Super rare.
- Similar to cystitis cystica.
Microscopic
Features:
- Cystic spaces lined by foveolar epithelium.
Ménétrier's disease
Gastric xanthoma
Gastric ischemia
- Gastric necrosis redirects here.
General
Microscopic
Features:
Image:
Portal hypertensive gastropathy
- Abbreviated PHG.
Amyloidosis of the stomach
- AKA gastric amyloidosis.
General
- Very rare.
- Etiologies: various - see amyloidosis.
Gross/endoscopy
- Red/swollen gastric folds.[19]
Endoscopic DDx:
Microscopic
Features:
- Lamina propria expanded by amorphous paucicellular material.
Image:
Stains
- Congo red stain +ve.
Eosinophilic gastritis
Proton pump inhibitor effect
- Abbreviated PPI effect.
Gastric polyps
Similar to colonic polyps - see intestinal polyps.
DDx polyp (similar to colon & rectum):
- Hyperplastic - most common, characterised by abundant elongated foveola + glands.
- Hamartomatous - weriod stuff.
- Inflammatory fibroid polyp - inflammation, myxoid stroma.
- Fundic gland polyp - cystic dilation, flat epithelium.
- Gastric adenoma - polypoid gastric dysplasia.
Inflammatory fibroid polyp
Hyperplastic polyp of the stomach
Fundic gland polyp
Neoplastic
The spectrum from benign to malignant is divided into five:[22]
- Benign.
- Indefinite for gastric epithelial dysplasia.
- Low-grade gastric epithelial dysplasia.
- High-grade gastric epithelial dysplasia.
- Gastric carcinoma.
Gastric dysplasia
- Gastric adenoma directs here.
- AKA gastric columnar dysplasia.
General
- Lesions that protrude into the lumen and are macroscopically apparent are known as: adenomas.[22]
- Polypoid forms are grouped various ways.[23]
Grading
Like in the colon - they are divided into:
- Low grade.
- High grade.
Subclassification
One subclassification:[24]
- Intestinal: goblet cells or Paneth cells.
- Not associated with FAP.
- Gastric: foveolar epithelium.
- Associated with familial adenomatous polyposis (FAP).
Microscopic
- Histologic criteria similar to columnar dysplasia in the esophagus.
- The threshold is much lower than in the colon and rectum.
Foveolar type
Features:
- Hyperchromasia at the surface - key feature.
- Cytoplasm with (shortened) champagne flute-like luminal aspect (apical mucin caps).
- Nuclear changes:
- Hyperchromasia.
- Enlargement.
- No intestinal metaplasia.
DDx:
Intestinal type
Features - intestinal:
- Intestinal metaplasia.
- Hyperchromasia of cytoplasm.
- Nuclear changes:
- Loss of nuclear polarity.
- Increased NC ratio.
- Elongation of nucleus and pseudostratification.
DDx:
Images
www:
- Gastric polyps - several images (sciencedirect.com).
- Gastric polyps - several images (achivesofpathology.org).
Grading
Low-grade gastric dysplasia
Features:
- Nuclear changes:
- Nuclear crowding/pseudostratification with hyperchromasia.
- Elongation of nuclei (cigar-shaped nuclei).
- Nuclear stratification intact; nuclei close to the basement membrane.
- Architecture:
- Focal irregularities in the glandular contours.
Negatives:
- No desmoplasia.
- No necrosis.
- No surface maturation.
DDx:
- Indefinite for dysplasia.
- High-grade gastric columnar dysplasia - see below.
- The threshold is much lower than in the colon and rectum!
Images:
- Low-grade gastric columnar dysplasia - several images (upmc.edu).
- Gastric low-grade dysplasia (nih.gov).[25]
High-grade gastric dysplasia
Features:
- Nuclear changes:
- Round hyperchromatic nuclei.
- Loss of normal nuclear stratification.
- Architecture:
- Irregularities in the glandular contours.
- Back-to-back glands.
- +/-Cribriforming of the glands.
- +/-Necrosis.
Negatives:
- No desmoplasia.
DDx:
- Low-grade gastric columnar dysplasia.
- Gastric adenocarcinoma.
Images
www:
- Gastric high-grade dysplasia - probably (nih.gov).[26]
- Gastric high-grade dysplasia - probably (nih.gov).
- Gastric high-grade dysplasia (nih.gov).[25]
Sign out
Indefinite for dypslasia
STOMACH, ANTRUM, BIOPSIES: - ANTRAL-TYPE MUCOSA INDEFINITE FOR DYSPLASIA WITH MODERATE CHRONIC INFLAMMATION. - EXTENSIVE INTESTINAL METAPLASIA. - NEGATIVE FOR HELICOBACTER-LIKE ORGANSIMS. - NEGATIVE FOR MALIGNANCY.
Intestinal type
STOMACH, ANTRUM, BIOPSIES: - ANTRAL-TYPE MUCOSA WITH FOCUS OF LOW-GRADE DYSPLASIA (INTESTINAL TYPE). - EXTENSIVE INTESTINAL METAPLASIA. - MODERATE CHRONIC INFLAMMATION. - NEGATIVE FOR HELICOBACTER-LIKE ORGANSIMS. - NEGATIVE FOR MALIGNANCY.
Foveolar type
STOMACH POLYP, EXCISION: - ADENOMATOUS POLYP, FOVEOLAR TYPE. - NEGATIVE FOR HIGH-GRADE DYSPLASIA. - NEGATIVE FOR HELICOBACTER-LIKE ORGANISMS.
Foveolar type with high-grade dysplasia
STOMACH POLYP, EXCISION: - LARGE ADENOMATOUS POLYP (FOVEOLAR TYPE) WITH HIGH-GRADE DYSPLASIA. - NEGATIVE FOR MALIGNANCY.
Gastric neuroendocrine tumour
- AKA neuroendocrine tumour of the stomach.
General
- Behaviour dependent on the subtype.
- Uncommon.
Overview of subtypes
Divided into four types:[27]
| Tumour type | Relative prevalence | Multifocality | Tumour size | Typical location | Clinical | Other | Histology |
|---|---|---|---|---|---|---|---|
| Type 1 | ~75% | yes | small (5-10 mm) | body | benign typically, female:male ~ 4:1, 50-60 years | chronic atrophic gastritis - usu. autoimmune | WDNET, WDNEC |
| Type 2 | rare | yes | small ~15 mm | body | aggressive, ~50 years old | assoc. MEN I, hyperchlorhydia | WDNEC, WDNET |
| Type 3 | 10-15% | no | small and large | variable location | aggressive if >2.0 cm, males > females | normal gastrin levels | WDNET |
| Type 4 | extremely rare | no | large | variable location | aggressive (mets usu. at time of Dx), males > females | elevated gastrin d/t parietal cell dysfunction | PDNEC |
Notes:
- WDNET = well-differentiated neuroendocrine tumour.
- WDNEC = well-differentiated neuroendocrine carcinoma.
- PDNEC = poorly-differentiated neuroendocrine carinoma.
Microscopic
Neoplastic rare
Gastric calcifying fibrous tumour
Gastric cancer
Gastric lymphoma
General
- Associated with helicobacter infection.[28]
- Usually MALT lymphoma (mucosa-associated lymphoid tissue lymphoma).
Microscopic
Features:
- Sheets of lymphoid cells.
- "Lymphoepithelial lesion" - gastric crypts invaded by a monomorphous population of lymphocytes.[29]
- Features:
- Cluster of lymphocytes - three cells or more - key feature.
- Single lymphocytes don't count.
- Clearing around the lymphocyte cluster.
- Cluster of lymphocytes - three cells or more - key feature.
- Associated with MALT lymphoma;[30] however, not specific.
- Features:
DDx:
IHC
- Panker -- most useful.
Others:
- CD3 (T cells) - scatter positivity.
- CD20 (B cells) +ve.
- CD138 (plasma cells).
- kappa, lambda -- often one is predominant, suggesting clonality.
- BCL2 +ve.
Treatment
- Triple therapy (two antibiotics, proton pump inhibitor (PPI)).[33]
- Surgery - if triple therapy fails.
Review paper: PMID 16950858.
Hereditary gastric cancer
Several syndromes are associated with gastric cancer:[34]
| Disease | Gene | Histology | Other |
|---|---|---|---|
| Hereditary diffuse gastric cancer (HDGC) syndrome | CDH1 (E-cadherin)[35] | diffuse - more specifically signet ring cell carcinoma | most important; assoc. invasive lobular carcinoma[36] |
| Lynch syndrome | MSH2, MLH1, others | ? | colorectal carcinoma, endometrial carcinoma |
| Familial adenomatous polyposis | APC | ? | adenomatous polyps |
| Peutz-Jeghers syndrome | STK11 | ? | stomach hamartomas - not precursor |
| Li-Fraumeni syndrome | TP53 (p53) | ? | AKA SBLA syndrome = sarcomas, breast, brain, leukemia, laryngeal, lung, adrenocortical carcinoma |
| Familial breast and ovarian cancer 2[37] | BRCA2 | ? | ? |
Gastric carcinoma
- Includes gastric adenocarcinoma.
See also
References
- ↑ ALS. 4 Feb 2009.
- ↑ Rubio, CA. (Jun 2007). "Gastric duodenal metaplasia in duodenal adenomas.". J Clin Pathol 60 (6): 661-3. doi:10.1136/jcp.2006.039388. PMC 1955048. PMID 16837629. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1955048/.
- ↑ Osborn M, Mazzoleni G, Santini D, Marrano D, Martinelli G, Weber K (1988). "Villin, intestinal brush border hydrolases and keratin polypeptides in intestinal metaplasia and gastric cancer; an immunohistologic study emphasizing the different degrees of intestinal and gastric differentiation in signet ring cell carcinomas". Virchows Arch A Pathol Anat Histopathol 413 (4): 303–12. PMID 2459839.
- ↑ Braunstein, EM.; Qiao, XT.; Madison, B.; Pinson, K.; Dunbar, L.; Gumucio, DL. (May 2002). "Villin: A marker for development of the epithelial pyloric border.". Dev Dyn 224 (1): 90-102. doi:10.1002/dvdy.10091. PMID 11984877.
- ↑ Sternberg H4P 2nd Ed., P.484
- ↑ URL: http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/git.htm. Accessed on: 3 December 2010.
- ↑ http://www.histology-world.com/stains/stains.htm
- ↑ Goggin N, Rowland M, Imrie C, Walsh D, Clyne M, Drumm B (December 1998). "Effect of Helicobacter pylori eradication on the natural history of duodenal ulcer disease". Arch. Dis. Child. 79 (6): 502-5. PMC 1717771. PMID 10210995. http://adc.bmj.com/cgi/pmidlookup?view=long&pmid=10210995.
- ↑ http://www.histology-world.com/stains/stains.htm
- ↑ http://www.histology-world.com/stains/stains.htm
- ↑ Malfertheiner, P.; Chan, FK.; McColl, KE. (Oct 2009). "Peptic ulcer disease.". Lancet 374 (9699): 1449-61. doi:10.1016/S0140-6736(09)60938-7. PMID 19683340.
- ↑ Lin J, McKenna BJ, Appelman HD (November 2010). "Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: a controlled study". Am. J. Surg. Pathol. 34 (11): 1672–7. doi:10.1097/PAS.0b013e3181f3de93. PMID 20962621.
- ↑ 13.0 13.1 Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 80. ISBN 978-0443066573.
- ↑ Kirsch R. 13 December 2010.
- ↑ URL: http://www.springerlink.com/content/u2v2525241754557/ Accessed on: 19 November 2010.
- ↑ Steen, S.; Lamont, J.; Petrey, L. (Jan 2008). "Acute gastric dilation and ischemia secondary to small bowel obstruction.". Proc (Bayl Univ Med Cent) 21 (1): 15-7. PMC 2190544. PMID 18209748. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2190544/.
- ↑ 17.0 17.1 Papanikolaou, IS.; Foukas, PG.; Sioulas, A.; Beintaris, I.; Panagopoulos, P.; Karamanolis, G.; Panayiotides, IG.; Dimitriadis, G. et al. (2011). "A case of gastric ischemic necrosis.". Endoscopy 43 Suppl 2 UCTN: E342. doi:10.1055/s-0030-1256795. PMID 22020717.
- ↑ Herman, J.; Chavalitdhamrong, D.; Jensen, DM.; Cortina, G.; Manuyakorn, A.; Jutabha, R. (Apr 2011). "The significance of gastric and duodenal histological ischemia reported on endoscopic biopsy.". Endoscopy 43 (4): 365-8. doi:10.1055/s-0030-1256040. PMID 21360426.
- ↑ 19.0 19.1 Kamata, T.; Suzuki, H.; Yoshinaga, S.; Nonaka, S.; Fukagawa, T.; Katai, H.; Taniguchi, H.; Kushima, R. et al. (2012). "Localized gastric amyloidosis differentiated histologically from scirrhous gastric cancer using endoscopic mucosal resection: a case report.". J Med Case Rep 6 (1): 231. doi:10.1186/1752-1947-6-231. PMC 3438062. PMID 22863214. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3438062/.
- ↑ Wu, D.; Lou, JY.; Chen, J.; Fei, L.; Liu, GJ.; Shi, XY.; Lin, HT. (Nov 2003). "A case report of localized gastric amyloidosis.". World J Gastroenterol 9 (11): 2632-4. PMID 14606114.
- ↑ Sawada, T.; Adachi, Y.; Akino, K.; Arimura, Y.; Ishida, T.; Ishii, Y.; Endo, T. (2012). "Endoscopic features of primary amyloidosis of the stomach.". Endoscopy 44 Suppl 2 UCTN: E275-6. doi:10.1055/s-0032-1309750. PMID 22814919.
- ↑ 22.0 22.1 Rugge, M.; Correa, P.; Dixon, MF.; Hattori, T.; Leandro, G.; Lewin, K.; Riddell, RH.; Sipponen, P. et al. (Feb 2000). "Gastric dysplasia: the Padova international classification.". Am J Surg Pathol 24 (2): 167-76. PMID 10680883.
- ↑ Park, do Y.; Lauwers, GY. (Apr 2008). "Gastric polyps: classification and management.". Arch Pathol Lab Med 132 (4): 633-40. doi:10.1043/1543-2165(2008)132[633:GPCAM]2.0.CO;2. PMID 18384215. http://www.archivesofpathology.org/doi/full/10.1043/1543-2165(2008)132%5B633:GPCAM%5D2.0.CO;2.
- ↑ URL: http://surgpathcriteria.stanford.edu/gitumors/gastric-adenoma/printable.html. Accessed on: 18 December 2012.
- ↑ 25.0 25.1 Kushima, R.; Kim, KM. (Sep 2011). "Interobserver Variation in the Diagnosis of Gastric Epithelial Dysplasia and Carcinoma between Two Pathologists in Japan and Korea.". J Gastric Cancer 11 (3): 141-5. doi:10.5230/jgc.2011.11.3.141. PMID 22076218.
- ↑ Correa, P.; Piazuelo, MB. (Jan 2012). "The gastric precancerous cascade.". J Dig Dis 13 (1): 2-9. doi:10.1111/j.1751-2980.2011.00550.x. PMID 22188910.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/StomachNET_11protocol.pdf. Accessed on: 29 March 2012.
- ↑ Mbulaiteye, SM.; Hisada, M.; El-Omar, EM. (2009). "Helicobacter Pylori associated global gastric cancer burden.". Front Biosci 14: 1490-504. PMID 19273142.
- ↑ Bailey, D. 6 August 2010.
- ↑ Papadaki, L.; Wotherspoon, AC.; Isaacson, PG. (Nov 1992). "The lymphoepithelial lesion of gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): an ultrastructural study.". Histopathology 21 (5): 415-21. PMID 1452124.
- ↑ Kim, K.; Kim, EJ.; Kim, MJ.; Song, HJ.; Lee, YS.; Jung, KW.; Yu, E. (Dec 2009). "Clinicopathological features of syphilitic gastritis in Korean patients.". Pathol Int 59 (12): 884-9. doi:10.1111/j.1440-1827.2009.02462.x. PMID 20021615.
- ↑ Long, BW.; Johnston, JH.; Wetzel, W.; Flowers, RH.; Haick, A. (Sep 1995). "Gastric syphilis: endoscopic and histological features mimicking lymphoma.". Am J Gastroenterol 90 (9): 1504-7. PMID 7661178.
- ↑ Zullo, A.; Hassan, C.; Andriani, A.; Cristofari, F.; De Francesco, V.; Ierardi, E.; Tomao, S.; Morini, S. et al. (Aug 2009). "Eradication therapy for Helicobacter pylori in patients with gastric MALT lymphoma: a pooled data analysis.". Am J Gastroenterol 104 (8): 1932-7; quiz 1938. doi:10.1038/ajg.2009.314. PMID 19532131.
- ↑ Sereno, M.; Aguayo, C.; Guillén Ponce, C.; Gómez-Raposo, C.; Zambrana, F.; Gómez-López, M.; Casado, E. (Sep 2011). "Gastric tumours in hereditary cancer syndromes: clinical features, molecular biology and strategies for prevention.". Clin Transl Oncol 13 (9): 599-610. PMID 21865131.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 192090
- ↑ Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R. et al. (Mar 1998). "E-cadherin germline mutations in familial gastric cancer.". Nature 392 (6674): 402-5. doi:10.1038/32918. PMID 9537325.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600185