Difference between revisions of "Diffuse lung diseases"
m (→Specimens) |
(→Diffuse alveolar damage: split out) |
||
| Line 101: | Line 101: | ||
==Diffuse alveolar damage== | ==Diffuse alveolar damage== | ||
*Abbreviated ''DAD''. | *Abbreviated ''DAD''. | ||
{{Main|Diffuse alveolar damage}} | |||
==Usual interstitial pneumonia== | ==Usual interstitial pneumonia== | ||
Revision as of 12:50, 18 April 2014
Diffuse lung diseases, abbreviated DLD, are a group of uncommon pathologies, also known as idiopathic interstitial pneumonias, abbreviated IIPs, and interstitial lung disease, abbreviated ILD.
They are a subgroup of the medical lung diseases.
Diffuse lung disease is the preferred term, as some diseases lumped into this group have involvement of the alveoli, i.e. are not interstitial. Also, some DLDs are not considered idiopathic, e.g. DIP is caused by smoking.
An introduction to pulmonary pathology is found in the pulmonary pathology article.
Overview
Specimens
- Diffuse lung disease is best assessed on an open biopsy.
- These procedure are quite invasive; thus, not infrequently clinicians will try a transbrochial biopsy that ends-up providing in useful information.
Sign out
LUNG, LEFT, TRANSBRONCHIAL BIOPSY: - SMALL FRAGMENTS OF LUNG PARENCHYMA WITHOUT SIGNIFICANT CHANGES, SEE MICROSCOPIC DESCRIPTION AND COMMENT. COMMENT: The clinical findings are noted. The size and type of biopsy significantly limit the interpretation. Suspected diffuse lung disease (interstitial lung disease) is best assessed on an open lung biopsy.
Micro
The sections show small fragments of lung parenchyma. A very small amount of anthracotic pigment is present. Interstitial fibrosis is not apparent. No granulomata are identified. No nuclear atypia is identified. Air-space macrophages show minimal pigmentation and are not present in large numbers. No significant airspace or interstitial inflammation is identified.
Histologic classification
- Can be complex,[1] and the combined efforts of clinicians, radiologists, and pathologists can help in the generation of a more specific diagnosis.[2][3]
Idiopathic interstitial pneumonia can be subclassified based on histologic appearance into the following patterns:[4][5]
| Histology | Clinical Correlates | Associations |
|---|---|---|
| Desquamative interstitial pneumonia (DIP) | DIP | Smoking |
| Diffuse alveolar damage (DAD) | ARDS, AIP, TRALI | ARDS: trauma, infection; TRALI: blood transfusion; AIP: viral (???) |
| Nonspecific interstitial pneumonia (NSIP) | NSIP | ??? |
| Respiratory bronchiolitis | RB-ILD | Smoking |
| Usual interstitial pneumonia (UIP) | CVD, IPF, drug toxicity, pneumoconiosis | Allergen (hypersensitivity pneumonitis), idiopathic, autoimmune |
| Organizing pneumonia | Cryptogenic organizing pneumonia | autoimmune (???) |
| Lymphocytic interstitial pneumonia (LIP) | LIP | Viral/autoimmune |
ARDS = adult respiratory distress syndrome, AIP = acute interstitial pneumonia, TRALI = transfusion related acute lung injury, CVD = collagen vascular disease, IPF = idiopathic pulmonary fibrosis.
Notes:
- Usual interstitial pneumonia is the most common type of ILD.[6]
Gross pathologic DDx by location
Causes of upper lung fibrosis FASSTEN:[7]
- Farmer's lung.
- Ankylosing spondylitis.
- Sarcoidosis.
- Silicosis.
- Tuberculosis (miliary).
- Eosinophilic granuloma.
- Neurofibromatosis.
Causes of lower lung fibrosis BAD RASH:[7]
- Bronchiolitis obliterans organizing pneumonia (BOOP).
- Asbestosis.
- Drugs (nitrofurantoin, hydralazine, isoniazid (INH), amiodarone).
- Rheumatologic disease.
- Aspiration.
- Scleroderma.
- Hamman-Rich syndrome (really should be -- idiopathic pulmonary fibrosis).
Note:
- Hamman-Rich syndrome is another name for acute interstitial pneumonia.[8]
Specific diseases/pattterns
Diffuse alveolar damage
- Abbreviated DAD.
Usual interstitial pneumonia
- Abbreviated UIP.
General
- It is sometimes used incorrectly as a synonym for idiopathic pulmonary fibrosis. It is a histomorphologic pattern and has a DDx (see below).
- UIP cannot be diagnosed via bronchoscopic or transbronchial biopsy,[9] as it is peripheral.
Epidemiology
- Disease of the old - rare in under 50 years old.[10]
- Dismal prognosis - mean survival after diagnosis ~ 2.8 years.[11]
Radiology
- Honeycombing - multiple defects that obliterate the normal lung architecture - multiple spherical voids in the lung parenchyma; radiologically these are seen as lucencies.[12]
- Usually subplural, i.e. peripheral lung.
- Classically lower lobe predominant.
- Traction bronchiectasis.
Note:
- Cysts - have thin walls (think of emphysema, lymphangioleiomyomatosis et cetera).
- Cysts may be isolated/not close to a neighbour.
- Medcyclopaedia defines it as: thin-walled, well-demarcated and >1 cm.[13]
Microscopic
Features:[14]
- Fibroblast foci:
- Interstitial inflammation.
- Microscopic honeycombing.
- Typically peripheral - cysts lined by ciliated epithelium.
- Spatial heterogeneity - patchy lesional distribution (areas of abnormal and normal lung may appear beside one another).
- Temporal heterogeneity - lesions of differing age side-by-side.[17]
Notes:
- Disease worse distant from large airways: lower lung field predominance, typically worse at periphery of lobule and lung.[18]
- Heterogeneity of inflammation: airspace macrophages & inflammation minimal in honeycombed foci.
DDx of UIP:[19]
- Idiopathic pulmonary fibrosis (UIP not otherwise specified).
- Asbestosis = UIP pattern + ferruginous bodies with asbestos fibers.
- Chronic hypersensitivity pneumonitis (AKA extrinsic allergic alveolitis) - classically centrilobular predominant +/- granulomas.
- Collagen vascular disease - includes systemic lupus erythematosus, rheumatoid arthritis, scleroderma.[20]
- Chronic drug toxicity.[21]
Asbestosis
Non-specific interstitial pneumonia
- Abbreviated NSIP.
General
- Better prognosis than UIP.
- Some radiologists and pathologists don't believe in this entity.
Associations:[17]
- Connective tissue disease.
- Rheumatoid arthritis.
Gross/Radiology
- No honeycombing.
- Fibrosis usually lower lung zone.
- Patchy ground glass.
Microscopic
Features:[17]
- Diffuse fibrosis:
- Uniform fibrosis (unlike UIP).
- "Linear fibrosis" has a good prognosis - should be mentioned in the report.
- Linear fibrosis = fibrosis that follows alveolar walls + no architectural distortion.
- +/-Lymphoid nodules - association with collagen vascular disease. (???)
- +/-Focal organizing pneumonia.
Notes:
- Inflammation in NSIP usually more prominent than in UIP.
- No honeycombing - key difference between UIP and NSIP.
DDx:
- Collagen vascular disease.
- Drug reaction.
- Hypersensitivity pneumonitis (extrinic allergic alveolitis).
- Lymphocytic interstitial pneumonia (LIP) - much more inflammation.
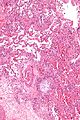
Organizing pneumonia
General
- Multiple causes, e.g. transplant rejection, infection.
Clinical diagnoses:[22]
- Transplant rejection.
- Cryptogenic organizing pneumonia (COP), AKA (idiopathic) bronchiolitis obliterans organizing pneumonia (BOOP).
- Should not be confused with constrictive bronchiolitis (AKA bronchiolitis obliterans).
- Collagen vascular disease.
- Toxic injury.
- Infection.
- Peri-tumor - in proximity to a space-occupying lesion (abscess, neoplasm).
Note:
- BOOP is used as a synonym for organizing pneumonia which has the long differential diagnosis above.[22]
- Confusingly, it may be used to refer to the idiopathic form of organizing pneumonia, now generally known as cryptogenic organizing pneumonia (COP).
- In other words, strictly speaking, BOOP is not the same as COP; idiopathic BOOP is COP.
- Confusingly, it may be used to refer to the idiopathic form of organizing pneumonia, now generally known as cryptogenic organizing pneumonia (COP).
Microscopic
Features:[23]
- Distal airway disease -- airways plugged with organizing exudate ("Masson bodies").
- "Organized exudate" = fluffy light-staining paucicellular regions with stellate cells (fibroblasts & immature connective tissue).
- No hobnailing of pneumocytes.
- Type 2 pneumocytes hyperplasia is absent.
DDx:
- Diffuse alveolar damage, proliferative phase - has type 2 pneumoncyte hyperplasia.
- Bronchiolitis obliterans.
Images
www:
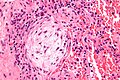
Hypersensitivity pneumonitis
- AKA extrinsic allergic alveolitis
General
- Exposure to stuffs... e.g. moldy hay - Farmer's lung, atypical mycobacteria - hot tub lung.
- Upper lung predominant disease.
- Mnemonic FASSTEN - upper lung field: Farmer's lung, Ankylosing spondylitis, Sarcoidosis, Silicosis, Tuberculosis (miliary), Eosinophilic granuloma, Neurofibromatosis.
- An immune complex-mediated hypersensitivity[25] (Gell-Coombs classification type III).
- If chronic, may develop into a cell-mediated hypersensitivity (Gell-Coombs classification type IV).[26]
Microscopic
Features:
- Lesions have centrilobular prominence - important feature. [27]
- Allergens enter lung through airway which has a centrilobular location.
- Granulomata (not typically seen in UIP) - important feature.[27]
- Chronic interstitial inflammation consisting primarily of lymphocytes.
- Interstitial fibrosis.
- Air space involvement (alveolitis).
DDx:
Images
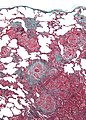
Lymphocytic interstitial pneumonia
- Often abbreviated LIP.
General
- Associated with autoimmune disorders (rheumatoid arthritis, pernicious anemia, Sjoegren syndrome)[28] and immunodeficiency.[29]
- Associated with viral infections (HIV, EBV, human T-cell leukemia virus (HTLV) type 1).
- Considered an AIDS-defining diagnosis.[30]
Gross
- Location: basilar predominance.
- Increased interstitial markings.
Microscopic
Features:[31]
- Small mature lymphocytes (usually B cells)[32] in the interstitium of the lung.
- Plasma cells.
- +/-Lymphoid follicles.
Negatives:
- No vasculitis.
- No necrosis.
Images:
DDx:
- Lymphoma.
- Follicular bronchitis/bronchiolitis.
- Nodular lymphoid hyperplasia.
- This is determined in part by radiology; it has nodules radiographically.
Stains
To exclude infection:[33]
- GMS stain -ve.
- Ziehl-Neelsen stain -ve.
IHC
- May be done to exclude lymphoma.
Respiratory bronchiolitis-interstitial lung disease
- Abbreviated RB-ILD or RBILD.
- AKA respiratory bronchiolitis-associated interstitial lung disease.
General
- May be an early version of DIP.
- Associated with smoking.
Microscopic
Features:[34]
- Brown pigmented airspace macrophages - smoker's macrophages - key feature.
- Inflammation of the terminal bronchioles.
Note:
- The histologic features of RBILD may be present peri-tumoural.
DDx:
Image:
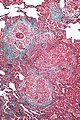
Desquamative interstitial pneumonia
- Abbreviated DIP.
General
Microscopic
Features:
- Abundant brown pigmented airspace macrophages - smoker's macrophages - key feature.
- Architecture preserved; "linear fibrosis".
Notes:
- Some fields of view may be indistinguishable from RBILD.
DDx:
- Amiodarone toxicity.
- Fibrotic NSIP.
Images:
Stains
- Macrophages PAS +ve.
See also
References
- ↑ Nicholson AG (November 2002). "Classification of idiopathic interstitial pneumonias: making sense of the alphabet soup". Histopathology 41 (5): 381-91. PMID 12405906. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0309-0167&date=2002&volume=41&issue=5&spage=381.
- ↑ Flaherty KR, King TE, Raghu G, et al (October 2004). "Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis?". Am. J. Respir. Crit. Care Med. 170 (8): 904-10. doi:10.1164/rccm.200402-147OC. PMID 15256390. http://ajrccm.atsjournals.org/cgi/pmidlookup?view=long&pmid=15256390.
- ↑ Kim DS, Collard HR, King TE (June 2006). "Classification and natural history of the idiopathic interstitial pneumonias". Proc Am Thorac Soc 3 (4): 285-92. doi:10.1513/pats.200601-005TK. PMID 16738191. http://pats.atsjournals.org/cgi/pmidlookup?view=long&pmid=16738191.
- ↑ Leslie KO, Wick MR. Practical Pulmonary Pathology: A Diagnostic Approach. Elsevier Inc. 2005. ISBN 978-0-443-06631-3.
- ↑ "American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001". Am. J. Respir. Crit. Care Med. 165 (2): 277-304. January 2002. PMID 11790668. http://ajrccm.atsjournals.org/cgi/pmidlookup?view=long&pmid=11790668.
- ↑ Visscher DW, Myers JL (June 2006). "Histologic spectrum of idiopathic interstitial pneumonias". Proc Am Thorac Soc 3 (4): 322-9. doi:10.1513/pats.200602-019TK. PMID 16738196. http://pats.atsjournals.org/cgi/pmidlookup?view=long&pmid=16738196.
- ↑ 7.0 7.1 Yeung, J.C.; Leonard, Blair J. N. (2005). The Toronto Notes 2005 - Review for the MCCQE and Comprehensive Medical Reference (2005 ed.). The Toronto Notes Inc. for Medical Students Inc.. pp. R13. ISBN 978-0968592854.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 90. ISBN 978-0781765275.
- ↑ Leslie, Kevin O.; Wick, Mark R. (2004). Practical Pulmonary Pathology: A Diagnostic Approach (1st ed.). Churchill Livingstone. pp. 186. ISBN 978-0443066313.
- ↑ AC UBC S.102.
- ↑ Bjoraker, JA.; Ryu, JH.; Edwin, MK.; Myers, JL.; Tazelaar, HD.; Schroeder, DR.; Offord, KP. (Jan 1998). "Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis.". Am J Respir Crit Care Med 157 (1): 199-203. PMID 9445300.
- ↑ http://www.medcyclopaedia.com/library/topics/volume_v_1/h/honeycombing.aspx
- ↑ http://www.medcyclopaedia.com/library/topics/volume_v_1/l/lung_cyst.aspx
- ↑ Leslie, Kevin O.; Wick, Mark R. (2004). Practical Pulmonary Pathology: A Diagnostic Approach (1st ed.). Churchill Livingstone. pp. 186-9. ISBN 978-0443066313.
- ↑ http://www.epler.com/IPFWhat%27sIPFDiseaseInformation2.htm
- ↑ Leslie, Kevin O.; Wick, Mark R. (2004). Practical Pulmonary Pathology: A Diagnostic Approach (1st ed.). Churchill Livingstone. pp. 189. ISBN 978-0443066313.
- ↑ 17.0 17.1 17.2 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 92. ISBN 978-0781765275.
- ↑ A. Churg. UBC S.103.
- ↑ Wick, Mark R.; Leslie, Kevin (2005). Practical pulmonary pathology: a diagnostic approach. Edinburgh: Churchill Livingstone. ISBN 0-443-06631-0. OCLC 156861539.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 374. ISBN 978-1416054542.
- ↑ Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC (2000). "Pulmonary drug toxicity: radiologic and pathologic manifestations". Radiographics : a review publication of the Radiological Society of North America, Inc 20 (5): 1245-59. PMID 10992015.
- ↑ 22.0 22.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 91. ISBN 978-0781765275.
- ↑ Klatt, Edward C. (2006). Robbins and Cotran Atlas of Pathology (1st ed.). Saunders. pp. 110. ISBN 978-1416002741.
- ↑ URL: http://150.59.224.157/pathology/index.php?first_category_id=2&second_category_id=20. Accessed on: 4 August 2011.
- ↑ Kaltreider, HB. (Nov 1993). "Hypersensitivity pneumonitis.". West J Med 159 (5): 570-8. PMID 8279154. http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/8279154/?tool=pubmed.
- ↑ URL: http://library.med.utah.edu/WebPath/EXAM/IMGQUIZ/pufrm.html. Accessed on: 4 December 2011.
- ↑ 27.0 27.1 Ohtani, Y.; Saiki, S.; Kitaichi, M.; Usui, Y.; Inase, N.; Costabel, U.; Yoshizawa, Y. (Aug 2005). "Chronic bird fancier's lung: histopathological and clinical correlation. An application of the 2002 ATS/ERS consensus classification of the idiopathic interstitial pneumonias.". Thorax 60 (8): 665-71. doi:10.1136/thx.2004.027326. PMID 16061708.
- ↑ URL: http://emedicine.medscape.com/article/299643-overview. Accessed on: 2 June 2010.
- ↑ Nicholson AG (August 2001). "Lymphocytic interstitial pneumonia and other lymphoproliferative disorders in the lung". Semin Respir Crit Care Med 22 (4): 409–22. doi:10.1055/s-2001-17384. PMID 16088689.
- ↑ Turner, BJ.; Eppes, S.; McKee, LJ.; Cosler, L.; Markson, LE. (Jan 1995). "A population-based comparison of the clinical course of children and adults with AIDS.". AIDS 9 (1): 65-72. PMID 7893443.
- ↑ URL: http://emedicine.medscape.com/article/299643-diagnosis. Accessed on: 2 June 2010.
- ↑ AFIP atlas of nontumour pathology. 2002. Vo. 2. P.265. ISBN 1-881041-79-4.
- ↑ URL: http://path.upmc.edu/cases/case176/dx.html. Accessed on: 8 January 2012.
- ↑ Leslie, Kevin O.; Wick, Mark R. (2004). Practical Pulmonary Pathology: A Diagnostic Approach (1st ed.). Churchill Livingstone. pp. 197-8. ISBN 978-0443066313.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 93. ISBN 978-0781765275.