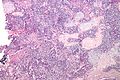
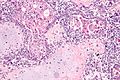
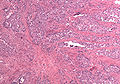
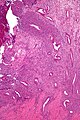
Uterine tumours
This article deals with uterine tumours, with the exception of the tumours that arise from the endometrium.
Uterine tumours are like water in the sea - very very common. Many hysterectomies are done for them. The most common are leiomyomata (AKA fibroids).
Pre-malignant endometrium and endometrial tumours are dealt with in the articles, endometrial hyperplasia and endometrial carcinoma.
Common benign
Uterine leiomyoma
- Often called fibroids.
- Fibroid uterus redirects here.
General
- Extremely common... 40% of women by age 40.
- Benign.
- Can be a cause of abnormal uterine bleeding (commonly abbreviated AUB).
- Large & multiple associated with infertility.
- May be treated medically with a selective progesterone receptor modulator, e.g. ulipristal (Fibristal).[1]
Gross
Feature:
- Sharply circumscribed.
- Gray-white.
- Whorled appearance.
Factor that raise concern for leiomyosarcoma:
- Haemorrhage.
- Cystic degeneration.
- Necrosis.
Microscopic
Features:
- Spindle cells arranged in fascicles.
- Fascicular appearance: adjacent groups of cells have their long axis perpendicular to one another; looks somewhat like a braided hair that was cut.
- Whorled arrangement of cells.
Negatives:
- Necrosis (low power) - suggestive of leiomyosarcoma.
- Hypercellularity.
- Nuclear atypia seen at low power.
- Few mitoses.
Images:
Variants
- Lipoleiomyoma - with adipose tissue.
- Image: Lipoleiomyoma - low mag. (WC).
- Hypercellular leiomyoma - hypercellularity associated with more mutations.[2]
- Atypical leiomyoma (AKA symplastic leiomyoma) - leiomyoma with nuclear atypia.
- Image: Atypical leiomyoma (WC).
- Benign metastasizing leiomyoma.[3]
- This is just what it sounds like. Some believe these are low grade leiomyosarcomas.
IHC
Work-up of suspicious leiomyomas:[4]
- CD10 +ve.[5]
- SMA +ve.
- Desmin +ve.
- Ki-67 -ve.
Others:
Sign out
UTERUS WITH CERVIX, UTERINE TUBES AND LEFT OVARY, TOTAL HYSTERECTOMY, BILATERAL SALPINGECTOMY AND LEFT OOPHRECTOMY: - LEIOMYOMATA WITH FOCAL CALCIFICATION AND HYALINE CHANGE. - SECRETORY PHASE ENDOMETRIUM. - LEFT OVARY WITHIN NORMAL LIMITS. - UTERINE TUBES WITHIN NORMAL LIMITS. - UTERINE CERVIX WITHIN NORMAL LIMITS.
Myomectomy
UTERINE MASSES ("FIBROIDS"), MYOMECTOMY:
- LEIOMYOMATA.
UTERINE MASS, HYSTEROSCOPIC MYOMECTOMY: - BENIGN SMOOTH MUSCLE FRAGMENTS COMPATIBLE WITH LEIOMYOMA. - SECRETORY PHASE ENDOMETRIUM.
Uncommon benign
Uterine adenofibroma
- AKA adenofibroma of the uterus.
General
- Uncommmon.
- Benign looking lesions can reoccur.[7]
- It has been proposed that these lesions are in fact well-differentiated adenosarcomas.[8]
Microscopic
Features:
- Moderately demarcated lesion with:
- Pale stroma and epithelioid/spindle cells.
- Simple cuboidal (or columnar) epithelium with eosinophilic cytoplasm.
- Low mitotic rate.
- Nuclear atypia minimal.
Note:
- Appearance similar to fibroadenoma.
DDx:
- Adenosarcoma.
Images:
- Adenofibroma of the uterus - low mag. (webpathology.com).
- Adenofibroma of the uterus - high mag. (webpathology.com).
Adenomatoid tumour
- Should not be confused with the bone tumour adamantinoma.
Uncertain malignant potential
Smooth muscle tumour of uncertain malignant potential
- Abbreviated STUMP.
General
- Like ASAP and ASCUS - a waffle category... when one isn't sure it is a leiomyoma vs. leiomyosarcoma.
- Clinical behaviour in uterus: usually benign.[9]
- Can be subclassified into four groups - as per Stanford.
- May be seen in the prostate gland.[10]
Management:
- Long-term follow-up.[9]
Microscopic
Features associated with recurrence:[9]
- Nuclear atypia.
DDx:
IHC
Features associated with recurrence:[9]
- p16 +ve.
- p53 +ve.
Malignant
Uterine carcinosarcoma
- AKA malignant mixed muellerian tumour, abbreviated MMMT.
General
- Associated with previous radiation exposure.
- Metstasize as adenocarcinoma.
- Aggressive/poor prognosis;[11] in one series 5 year survival ~= 30-35%.[12]
- Considered to be a poorly differentiated endometrial carcinoma with metaplastic changes.[13]
- Case reports of MMMT in ovary and fallopian tube.
Microscopic
Features:[14]
- Biphasic tumour:
- Malignant glandular component (adenocarcinoma).
- Malignant stromal component (one of the following):
- Homologous type (tissue native to uterus):
- Smooth muscle (leiomyosarcoma).
- Fibrous tissue (fibrosarcoma).
- Heterologous type (tissue not native to the uterus):
- Skeletal muscle (rhabdomyosarcoma).
- Cartilage (chondrosarcoma).
- Bone (osteosarcoma).
- Undifferentiated sarcoma (pleomorphic undifferentiated sarcoma).
- Homologous type (tissue native to uterus):
DDx:
Images
www:
Adenosarcoma of the uterus
- AKA uterine adenocarcinoma.
General
Features:[15]
- Uncommon.
- May prolapse through cervical os and thus present as cervical polyp.
- Most commonly uterine corpus, occasionally cervix and ovary, rarely in the vagina, fallopian tube, peritoneal surfaces, intestine.
- Typically 30-40 years old.
Clinical:[16]
- Most common presentations of Müllerian adenosarcoma (percentages based on series of 41 individuals[17]):
- Vaginal bleeding ~ 70%.
- Pelvic mass ~ 40%.
- Uterine polyp ~ 30%.
- Prognosis (based on series of ~500 individuals[18]):
- Favourable outcome - most detected at an early stage.
- ~80% five year survival for stage I tumours.
- Outcome better than carcinosarcoma.
- Favourable outcome - most detected at an early stage.
Treatment:
- TAH-BSO.
- Tumours are estrogen responsive.
- Chemotherapy (platin-based).[17]
Microscopic
- "Malignant stroma" - key feature.
- Benign glands with an abnormal shape.
- "Cambium layer" = increased cellularity around the epithelial elements.[15][20]
Notes:
- Tumour may vaguely resemble a phyllodes tumour.[15]
- Cambium layer - seen in: adenosarcoma, botryoid RMS.[20]
DDx:
Images
IHC
- CD10 +ve.[15]
- ER +ve.
- PR +ve.
Uterine leiomyosarcoma
General
- Poor prognosis.
- Do not (generally) arise from leiomyomas.
- Often singular, i.e. one tumour; unlike leiomyomas (which are often multiple).
Gross
Features:
- "Fleshy" appearance.
- Necrosis.
- Large size.
- Often singular, i.e. one lesion; leiomyomata are often multiple.
Microscopic
Features:
- Smooth muscle differentiation - essential.
- Fascicular architecture.
- Whorled look at low power.
- Groups of spindle cells cut peripendicular to their long axis adjacent to groups of spindle cells cut in the plane of their long axis.
- May rely on IHC - if poorly differentiated.
- Fascicular architecture.
- Malignant histomorphologic features - two of three required - key features:[21]
- Nuclear pleomorphism.
- Coagulative tumour cell necrosis
- Should be patchy/multifocal.
- Zonal necrosis is suggestive of vascular cause and may be a degenerative change.
- Zonal necrosis may be seen in (benign) leiomyomas.
- Mitoses.
- 10 mitoses/HPF.
- 5 mitoses/HPF - if epithelioid.
- 2 mitoses/HPF - if myxoid.
Note:
- The mitotic rate seems to be a relatively weak predictor; a modest rate may be malignant and a high rate benign.[22]
DDx:
- Endometrial stromal sarcoma.
- Uterine carcinosarcoma.
- Undifferentiated endometrial sarcoma.
- Smooth muscle tumour of uncertain malignant potential.
- Uterine leiomyoma.
IHC
- CD10 -ve.
- Positive for SMC markers.
- Desmin - present in all three types of muscle.
- Caldesmon.
- Smooth muscle myosin.
- p16 +ve.[6]
- Useful for differentiation from leiomyoma.
Endometrial stromal tumours
This grouping includes the gamut from benign to malignant.
Overview
WHO classification:[23]
- Endometrial stromal nodule - not a tumour.
- Endometrial stromal sarcoma (ESS), low grade.
- Undifferentiated endometrial sarcoma (UES).
Notes:
- Some believe in a "high grade ESS"... some don't.[24]
Endometrial stromal nodule
- Abbreviated ESN.
General
- Benign.
Microscopic
Features:
- Well-circumscribed - key feature.
- No vascular invasion.
DDx:
- Endometrial stromal sarcoma (ESS), UES - myometrial invasion or vascular invasion.
Images:
Endometrial stromal sarcoma
- Abbreviated ESS.
- Previously known as low-grade endometrial stromal sarcoma.
Undifferentiated endometrial sarcoma
- Abbreviated as UES.
- Previously known as high-grade endometrial stromal sarcoma.[26]
General
- Malignant.
- Rare.
- This can be thought of as pleomorphic undifferentiated sarcoma in the uterus.
Microscopic
Features:
- Marked nuclear atypia.
- Mitoses+++.
- Poorly differentiated - key feature
- Looks nothing like low grade endometrial stromal sarcoma.
- Negative for smooth muscle markers (to exclude leiomyosarcoma).
Notes:
- Need IHC to diagnose.
DDx:
IHC
Features:[27]
- SMA ~50% +ve.
Typically negative:[27]
- Smooth muscle markers: desmin, h-caldesmon.
- Skeletal muscle markers: Myf4, actin.
- Melanoma: S100, HMB-45.
- GIST: CD117.
Weird stuff
Trophoblastic tumours
Uterine tumour resembling an ovarian sex cord stromal tumour
- Abbreviated UTROSCT.
Atypical polypoid adenomyoma of the uterus
- Abbreviated APA.
- AKA atypical polypoid adenomyoma.
See also
References
- ↑ Delev, DP.. "Ulipristal acetate--a review of the new therapeutic indications and future prospects.". Folia Med (Plovdiv) 55 (3-4): 5-10. PMID 24712276.
- ↑ Pandis, N.; Heim, S.; Willén, H.; Bardi, G.; Flodérus, U-M.; Mandahl, N.; Mitelman, F. (Jan 1991). "Histologic—cytogenetic correlations in uterine leiomyomas.". International Journal of Gynecological Cancer 1 (4): 163-68. http://www3.interscience.wiley.com/journal/119360394/abstract.
- ↑ Patton, KT.; Cheng, L.; Papavero, V.; Blum, MG.; Yeldandi, AV.; Adley, BP.; Luan, C.; Diaz, LK. et al. (Jan 2006). "Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis.". Mod Pathol 19 (1): 130-40. doi:10.1038/modpathol.3800504. PMID 16357844. http://www.nature.com/modpathol/journal/v19/n1/full/3800504a.html.
- ↑ STC. 25 February 2009.
- ↑ 5.0 5.1 Zhu, XQ.; Shi, YF.; Cheng, XD.; Zhao, CL.; Wu, YZ. (Jan 2004). "Immunohistochemical markers in differential diagnosis of endometrial stromal sarcoma and cellular leiomyoma.". Gynecol Oncol 92 (1): 71-9. PMID 14751141.
- ↑ 6.0 6.1 Gannon, BR.; Manduch, M.; Childs, TJ. (Jan 2008). "Differential Immunoreactivity of p16 in leiomyosarcomas and leiomyoma variants.". Int J Gynecol Pathol 27 (1): 68-73. doi:10.1097/pgp.0b013e3180ca954f. PMID 18156978.
- ↑ Seltzer, VL.; Levine, A.; Spiegel, G.; Rosenfeld, D.; Coffey, EL. (Jun 1990). "Adenofibroma of the uterus: multiple recurrences following wide local excision.". Gynecol Oncol 37 (3): 427-31. PMID 2351327.
- ↑ Gallardo, A.; Prat, J. (Feb 2009). "Mullerian adenosarcoma: a clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma.". Am J Surg Pathol 33 (2): 278-88. doi:10.1097/PAS.0b013e318181a80d. PMID 18941402.
- ↑ 9.0 9.1 9.2 9.3 Ip PP, Cheung AN, Clement PB (July 2009). "Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases". Am. J. Surg. Pathol. 33 (7): 992–1005. doi:10.1097/PAS.0b013e3181a02d1c. PMID 19417585.
- ↑ Nagar, M.; Epstein, JI. (Jun 2011). "Epithelial proliferations in prostatic stromal tumors of uncertain malignant potential (STUMP).". Am J Surg Pathol 35 (6): 898-903. doi:10.1097/PAS.0b013e318214f2f2. PMID 21572264.
- ↑ Ivy, JJ.; Unger, JB.. "Malignant mixed mullerian sarcomas of the uterus--the LSUHSC Shreveport experience.". J La State Med Soc 156 (6): 324-6. PMID 15688674.
- ↑ Callister, M.; Ramondetta, LM.; Jhingran, A.; Burke, TW.; Eifel, PJ. (Mar 2004). "Malignant mixed Müllerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome.". Int J Radiat Oncol Biol Phys 58 (3): 786-96. doi:10.1016/S0360-3016(03)01561-X. PMID 14967435.
- ↑ D'Angelo, E.; Prat, J. (Jan 2010). "Uterine sarcomas: a review.". Gynecol Oncol 116 (1): 131-9. doi:10.1016/j.ygyno.2009.09.023. PMID 19853898.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 428. ISBN 978-0781765275.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 McCluggage, WG. (Mar 2010). "Mullerian adenosarcoma of the female genital tract.". Adv Anat Pathol 17 (2): 122-9. doi:10.1097/PAP.0b013e3181cfe732. PMID 20179434.
- ↑ Abu, J.; Ireland, D.; Brown, L. (Apr 2007). "Adenosarcoma of an endometrial polyp in a 27-year-old nulligravida: a case report.". J Reprod Med 52 (4): 326-8. PMID 17506376.
- ↑ 17.0 17.1 Verschraegen, CF.; Vasuratna, A.; Edwards, C.; Freedman, R.; Kudelka, AP.; Tornos, C.; Kavanagh, JJ.. "Clinicopathologic analysis of mullerian adenosarcoma: the M.D. Anderson Cancer Center experience.". Oncol Rep 5 (4): 939-44. PMID 9625851.
- ↑ Arend, R.; Bagaria, M.; Lewin, SN.; Sun, X.; Deutsch, I.; Burke, WM.; Herzog, TJ.; Wright, JD. (Nov 2010). "Long-term outcome and natural history of uterine adenosarcomas.". Gynecol Oncol 119 (2): 305-8. doi:10.1016/j.ygyno.2010.07.001. PMID 20688363.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1089. ISBN 0-7216-0187-1.
- ↑ 20.0 20.1 URL: http://www.medilexicon.com/medicaldictionary.php?t=48297. Accessed on: 9 August 2011.
- ↑ Ip, PP.; Cheung, AN. (Dec 2011). "Pathology of uterine leiomyosarcomas and smooth muscle tumours of uncertain malignant potential.". Best Pract Res Clin Obstet Gynaecol 25 (6): 691-704. doi:10.1016/j.bpobgyn.2011.07.003. PMID 21865091.
- ↑ Guo, L.; Liu, T.; Huang, H. (Oct 1996). "[Reappraisal of the pathological criteria for uterine leiomyosarcoma].". Zhonghua Bing Li Xue Za Zhi 25 (5): 266-9. PMID 9388868.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 426. ISBN 978-0781765275.
- ↑ Amant F, Vergote I, Moerman P (November 2004). "The classification of a uterine sarcoma as 'high-grade endometrial stromal sarcoma' should be abandoned". Gynecol. Oncol. 95 (2): 412–3; author reply 413. doi:10.1016/j.ygyno.2004.07.021. PMID 15491769. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WG6-4DF46J8-3&_user=1166899&_coverDate=11%2F01%2F2004&_rdoc=1&_fmt=high&_orig=search&_sort=d&_docanchor=&view=c&_searchStrId=1204975755&_rerunOrigin=google&_acct=C000051839&_version=1&_urlVersion=0&_userid=1166899&md5=d6ec1eee2941460a085d1dac6615b5a5.
- ↑ Baker, P.; Oliva, E. (Mar 2007). "Endometrial stromal tumours of the uterus: a practical approach using conventional morphology and ancillary techniques.". J Clin Pathol 60 (3): 235-43. doi:10.1136/jcp.2005.031203. PMID 17347285. http://jcp.bmj.com/content/60/3/235.full.
- ↑ Feng, W.; Malpica, A.; Skaland, I.; Gudlaugsson, E.; Robboy, SJ.; Dalen, I.; Hua, K.; Zhou, X. et al. (2013). "Can proliferation biomarkers reliably predict recurrence in world health organization 2003 defined endometrial stromal sarcoma, low grade?". PLoS One 8 (10): e75899. doi:10.1371/journal.pone.0075899. PMID 24146786.
- ↑ 27.0 27.1 Abeler, VM.; Nenodovic, M. (May 2011). "Diagnostic immunohistochemistry in uterine sarcomas: a study of 397 cases.". Int J Gynecol Pathol 30 (3): 236-43. doi:10.1097/PGP.0b013e318200caff. PMID 21464730.