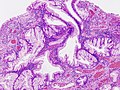
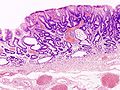
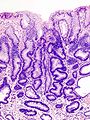
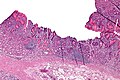
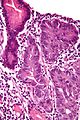
Stomach
Stomach is an important organ for pathologists. It is often inflamed and may be a site that cancer arises from. Gastroenterologists often biopsy the organ. Surgeon take-out the organ. It connects the esophagus to the duodenum. An introduction to gastrointestinal pathology is in the gastrointestinal pathology article.
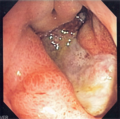
Normal stomach
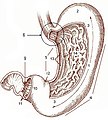
Gross anatomy
- Cardia - first part of the stomach; joins with esophagus.
- Fundus - superior portion - not attached directly to the esophagus.
- Body - contains parietal cells.
- Pylorus - distal (think pyloric stenosis); it joins with the duodenum.
- AKA antrum.
Image
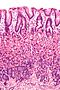
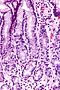
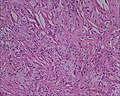
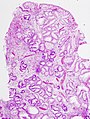
Microscopic
Foveolar cells versus intestinal goblet cells
- Intestinal goblet cells - clear mucin.
- Foveolar cells - eosinophilic contents.
Stomach versus intestine
A tabular comparison:[1]
| Feature | Intestine | Stomach |
|---|---|---|
| Spacing | Goblets cell - spaced | Foveolar cells - beside one another |
| Morphology of epithelial cells | columnar | tall columnar (Champagne flute) |
| Vesicle at luminal surface | touching/small opening | wide open |
| PAS-D | -ve (???) | +ve[2] |
| Villin stain[3][4] | +ve | -ve |
| Images | Tubular adenoma - goblet cells on right of image (WC) |
Gastric biopsy (microscopy-uk.org.uk), Stomach with cancer - PAS (WC), Stomach (WC) |
Notes:
- Intraepithelial lymphocytes in the gastric mucosa have a clear halo around 'em.[5]
- Memory device: Folveolar cells have friends, i.e. they are close to other foveolar cells.
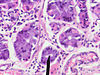
Gastric antrum versus gastric body
| Cell | Body | Antrum | Histology | Image |
|---|---|---|---|---|
| Parietal cell | abundant | few or none | parietal cells: intensely eosinophilic cytoplasm |
|
| Chief cell | present | absent | chief cells: basophilic cytoplasm, IHC: +ve for pepsinogen I |
|
| G cell | absent | present | fried egg appearance (clear cytoplasm, round nucleus); look at high power - usu. middle 1/3 of gland,[6] IHC: +ve for gastrin. |
|
| Surface | flat | blunted villi | antrum is somewhat duodenum-like |
|
| Gastric glands / mucosa |
thick | thin | not so useful for discrimination |
body - thick, body & antrum |
Notes:
- G cells may superficially resemble intraepithelial lymphocytes.
- G cell nucleus is usu. perfectly round and slightly larger (diameter of 12 micrometers?) than a lymphocyte nucleus (diameter ~ 9-10 micrometers?).
Sign out
Short version
STOMACH, BIOPSY: - BODY AND ANTRAL-TYPE GASTRIC MUCOSA WITHIN NORMAL LIMITS.
STOMACH, BIOPSY: - BODY AND ANTRAL-TYPE GASTRIC MUCOSA WITHIN NORMAL LIMITS. - NEGATIVE FOR HELICOBACTER-LIKE ORGANISMS.
STOMACH, BIOPSY: - ANTRAL-TYPE GASTRIC MUCOSA WITHIN NORMAL LIMITS. - NEGATIVE FOR HELICOBACTER-LIKE ORGANISMS.
Long version
STOMACH, BIOPSY: - BODY/ANTRAL-TYPE GASTRIC MUCOSA. - INFLAMMATION: ABSENT. - ATROPHY: ABSENT. - INTESTINAL METAPLASIA: ABSENT. - HELICOBACTER-LIKE ORGANISMS: NOT IDENTIFIED WITH ROUTINE STAINS. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY.
Sleeve gastrectomy
- Indication: morbid obesity.
STOMACH, GREATER CURVE, SLEEVE GASTRECTOMY: - STOMACH WALL WITHIN NORMAL LIMITS.
Introduction
Useful stains for stomach
- Cresyl violet stain[7] - used to find H. pylori.[8]
- Alcian blue stain - used to find mucin[9] which is present in intestinal metaplasia
Things to look for...
- Parietal cells (indicate you're in the body of the stomach) - pink (eosinophilic) cytoplasm.
- Lack of parietal cells -- DDx: Bx of antrum (pylorus), Bx of cardia, pernicious anemia.
- Goblet cells = intestinal metaplasia.
- Architectural distortion of gastric glands - suspect cancer.
- Signet ring cells = (usually) gastric carcinoma.
- Can be very easy to miss in some biopsies.
- Inflammation + small bacteria = suspect H. pylori gastritis.
Some patterns
Gastric atrophy
General
- Has a wide differential diagnosis.
Microscopic
Can take three general forms:
- Intestinal metaplasia - see intestinal metaplasia section.
- Pseudopyloric metaplasia; gastric body looks like gastric antrum.
- Characterized by foveolar hyperplasia.
- Cell loss without replacement.
- Clue is deep inflammation in the body.
Plasma cells in the stomach
DDx of plasmacytosis:
- Plasma cell neoplasm.
- Syphilis.
- Chronic gastritis.
Granulomatous gastritis
- Usual DDx of granulomatous disease (see Basics article):
- DNF AAII:
- Drugs, Neoplasms, Foreign body, Autoimmune, Allergic, Infectious, Idiopathic.
- DNF AAII:
Important ones:
- Autoimmune - Crohn's disease.
- Infectious - Tuberculosis.
- Idiopathic - Sarcoidosis.
Non-neoplastic disease
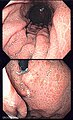
Peptic ulcer disease
- Abbreviated PUD.
- For duodenal manifestations see Peptic duodenitis.
General
- Benign.
Complications:
- Hemorrhage.
- Obstruction.
- Perforation - can be fatal.
Etiology - typically:[11]
Gross
Features:
- Typically in the duodenum; duodenum:stomach = ~4:1.
- Epithelial defect with punched-out edges (suggestive of a benign process).
Note:
- Heaped edges - suggestive of cancer.
Endoscopic image
Microscopic
Features:
- Loss of epithelium.
- Inflammation.
- +/-Helicobacter organisms - see Helicobacter gastritis.
Gastritis
Helicobacter gastritis
Intestinal metaplasia of the stomach
Inflammatory bowel disease & the stomach
- Histopathologic findings are usually non-specific.
- Conventional thinking was upper GI involvement = Crohn's disease; this is changing.[12]
Microscopic
Features:[13]
- Focal inflammation.
- Common finding - non-specific.
- +/-Granulomas.
Miscellaneous
This is a grab bag of stuff seen in the stomach. Some of it is quite rare.
Gastric antral vascular ectasia
Reactive gastropathy
Autoimmune metaplastic atrophic gastritis
- AKA autoimmune gastritis.
Collagenous gastritis
General
- Very rare.
- Associated with collagenous colitis.
Microscopic
Features:
- Eosinophilic material (collagen) expands lamina propria.
- Band of collagen must be ~thick as RBC diameter.
- Proven by trichrome stain that highlights collagen.
- Band of collagen must be ~thick as RBC diameter.
DDx:
Gastritis cystitis profunda
- AKA Gastritic cystica profunda.[citation needed]
General
- May be associated with glandular proliferation as well.[14] (???)
- Super rare.
- Similar to cystitis cystica.
Microscopic
Features:
- Cystic spaces lined by foveolar epithelium.
Ménétrier's disease
Gastric xanthoma
General
- Uncommon.
- Benign.
Gross/endoscopic
Microscopic
Features:[15]
- Collections of gastric lamina propria with lipid-laden macrophages.
DDx:
- Signet ring cell carcinoma.[16]
- Whipple disease.
- MAC infection.
Images
www:
IHC
- CD68 +ve.
- Panker (AE1/AE3) -ve.
Gastric ischemia
- Gastric necrosis redirects here.
General
Microscopic
Features:
Image:
Portal hypertensive gastropathy
- Abbreviated PHG.
General
- Due to portal hypertension.
- Usually secondary to liver cirrhosis which is typically due to alcoholism.
- Reported in approximately 65% of cirrhotics with portal hypertension in one paper.[20]
- Usually secondary to liver cirrhosis which is typically due to alcoholism.
Gross
Features:[21]
- Mosaic-like pattern.
- May be referred to as snakeskin-like pattern.[22]
- Usu. body of stomach.
- +/-Red spots.
Note:
- May mimic eosinophilic gastritis.[22]
Images
Microscopic
Features:[23]
- Dilated capillaries in the submucosa (prominent) and to a lesser extent in the lamina propria - key feature.
Notes:
- May be associated with hyperplastic-like polyps.[24]
- Subepithelial granulation tissue and vascular proliferation.
- Findings in mucosal biopsies are often nonspecific, i.e. not diagnostic.[23]
DDx:
- Gastric antral vascular ectasia - have thrombi in the dilated blood vessels.
Sign out
STOMACH, BIOPSY: - ANTRAL-TYPE AND BODY-TYPE GASTRIC MUCOSA WITH PROMINENT CAPILLARIES AND MODERATE CHRONIC INACTIVE INFLAMMATION. - NEGATIVE FOR HELICOBACTER-LIKE ORGANISMS. - NEGATIVE FOR INTESTINAL METAPLASIA. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY. COMMENT: No fibrin thrombi are seen. The findings are compatible with portal hypertension. Clinical correlation is required.
Amyloidosis of the stomach
- AKA gastric amyloidosis.
General
- Very rare.
- Etiologies: various - see amyloidosis.
Gross/endoscopy
- Red/swollen gastric folds.[25]
Endoscopic DDx:
Microscopic
Features:
- Lamina propria expanded by amorphous paucicellular material.
Image:
Stains
- Congo red stain +ve.
Gastric polyps
Similar to colonic polyps - see intestinal polyps.
DDx polyp (similar to colon & rectum):
- Hyperplastic - most common, characterised by abundant elongated foveola + glands.
- Hamartomatous - weriod stuff.
- Inflammatory fibroid polyp - inflammation, myxoid stroma.
- Fundic gland polyp - cystic dilation, flat epithelium.
- Gastric adenoma - polypoid gastric dysplasia.
Inflammatory fibroid polyp
Hyperplastic polyp of the stomach
- AKA gastric hyperplastic polyp.
General
- Benign.
- Most common gastric polyp.[28]
Microscopic
Features:[29]
- Abundant foveolar cells and elongated glands - key feature.
- +/-Gland dilation.
Negatives:
- No atypical nuclei.
- No hyperchromasia.
- No loss of pseudostratification.
Notes:
- No serrations - as in the colon.
DDx:
- Ménétrier's disease[30] (hyperplastic hypersecretory gastropathy).
- Juvenile polyp[28] - abundant lamina propria, dilated glands may have neutrophils.[31]
- Peutz-Jeghers polyp - thick superficial muscle.
- Fundic gland polyp - doesn't have foveolar hyperplasia.
Images
www:
Sign out
POLYP, STOMACH (ANTRUM), EXCISION: - HYPERPLASTIC POLYP.
Micro
The sections show antral-type gastric mucosa with hyperplastic gastric pits. No gland dilation is apparent. The epithelium matures to the surface. The lamina propria is not expanded.
Focal neutrophilic inflammation is present. No Helicobacter-like organisms are identified. No intestinal metaplasia is present. No mitotic activity or nuclear atypia is apparent.
Fundic gland polyp
Neoplastic
The spectrum from benign to malignant is divided into five:[32]
- Benign.
- Indefinite for gastric epithelial dysplasia.
- Low-grade gastric epithelial dysplasia.
- High-grade gastric epithelial dysplasia.
- Gastric carcinoma.
Gastric dysplasia
- Gastric adenoma directs here.
- AKA gastric columnar dysplasia.
General
- Lesions that protrude into the lumen and are macroscopically apparent are known as: adenomas.[32]
- Polypoid forms are grouped various ways.[30]
Grading
Like in the colon - they are divided into:
- Low grade.
- High grade.
Subclassification
One subclassification:[33]
- Intestinal: goblet cells or Paneth cells.
- Not associated with FAP.
- Gastric: foveolar epithelium.
- Associated with familial adenomatous polyposis (FAP).
Microscopic
- Histologic criteria similar to columnar dysplasia in the esophagus.
- The threshold is much lower than in the colon and rectum.
Foveolar type
Features:
- Hyperchromasia at the surface - key feature.
- Cytoplasm with (shortened) champagne flute-like luminal aspect (apical mucin caps).
- Nuclear changes:
- Hyperchromasia.
- Enlargement.
- No intestinal metaplasia.
DDx:
Intestinal type
Features - intestinal:
- Intestinal metaplasia.
- Hyperchromasia of cytoplasm.
- Nuclear changes:
- Loss of nuclear polarity.
- Increased NC ratio.
- Elongation of nucleus and pseudostratification.
DDx:
Images
www:
- Gastric polyps - several images (sciencedirect.com).
- Gastric polyps - several images (achivesofpathology.org).
Grading
Low-grade gastric dysplasia
Features:
- Nuclear changes:
- Nuclear crowding/pseudostratification with hyperchromasia.
- Elongation of nuclei (cigar-shaped nuclei).
- Nuclear stratification intact; nuclei close to the basement membrane.
- Architecture:
- Focal irregularities in the glandular contours.
Negatives:
- No desmoplasia.
- No necrosis.
- No surface maturation.
DDx:
- Indefinite for dysplasia.
- High-grade gastric columnar dysplasia - see below.
- The threshold is much lower than in the colon and rectum!
Images:
- Low-grade gastric columnar dysplasia - several images (upmc.edu).
- Gastric low-grade dysplasia (nih.gov).[34]
High-grade gastric dysplasia
Features:
- Nuclear changes:
- Round hyperchromatic nuclei.
- Loss of normal nuclear stratification.
- Architecture:
- Irregularities in the glandular contours.
- Back-to-back glands.
- Cribriforming of the glands.
- +/-Necrosis.
Negatives:
- No desmoplasia.
DDx:
- Low-grade gastric columnar dysplasia.
- Gastric adenocarcinoma.
Images
www:
- Gastric high-grade dysplasia - probably (nih.gov).[35]
- Gastric high-grade dysplasia - probably (nih.gov).
- Gastric high-grade dysplasia (nih.gov).[34]
Sign out
Indefinite for dypslasia
STOMACH, ANTRUM, BIOPSIES: - ANTRAL-TYPE MUCOSA INDEFINITE FOR DYSPLASIA WITH MODERATE CHRONIC INFLAMMATION. - EXTENSIVE INTESTINAL METAPLASIA. - NEGATIVE FOR HELICOBACTER-LIKE ORGANSIMS. - NEGATIVE FOR MALIGNANCY.
Intestinal type
STOMACH, ANTRUM, BIOPSIES: - ANTRAL-TYPE MUCOSA WITH FOCUS OF LOW-GRADE DYSPLASIA (INTESTINAL TYPE). - EXTENSIVE INTESTINAL METAPLASIA. - MODERATE CHRONIC INFLAMMATION. - NEGATIVE FOR HELICOBACTER-LIKE ORGANSIMS. - NEGATIVE FOR MALIGNANCY.
Foveolar type
STOMACH POLYP, BIOPSY: - ADENOMATOUS POLYP, FOVEOLAR TYPE. - NEGATIVE FOR HIGH-GRADE DYSPLASIA. - NEGATIVE FOR HELICOBACTER-LIKE ORGANISMS.
Gastric neuroendocrine tumour
- AKA neuroendocrine tumour of the stomach.
General
- Behaviour dependent on the subtype.
- Uncommon.
Overview of subtypes
Divided into four types:[36]
| Tumour type | Relative prevalence | Multifocality | Tumour size | Typical location | Clinical | Other | Histology |
|---|---|---|---|---|---|---|---|
| Type 1 | ~75% | yes | small (5-10 mm) | body | benign typically, female:male ~ 4:1, 50-60 years | chronic atrophic gastritis - usu. autoimmune | WDNET, WDNEC |
| Type 2 | rare | yes | small ~15 mm | body | aggressive, ~50 years old | assoc. MEN I, hyperchlorhydia | WDNEC, WDNET |
| Type 3 | 10-15% | no | small and large | variable location | aggressive if >2.0 cm, males > females | normal gastrin levels | WDNET |
| Type 4 | extremely rare | no | large | variable location | aggressive (mets usu. at time of Dx), males > females | elevated gastrin d/t parietal cell dysfunction | PDNEC |
Notes:
- WDNET = well-differentiated neuroendocrine tumour.
- WDNEC = well-differentiated neuroendocrine carcinoma.
- PDNEC = poorly-differentiated neuroendocrine carinoma.
Microscopic
Neoplastic rare
Gastric calcifying fibrous tumour
Gastric cancer
Gastric lymphoma
General
- Associated with helicobacter infection.[37]
- Usually MALT lymphoma (mucosa-associated lymphoid tissue lymphoma).
Microscopic
Features:
- Sheets of lymphoid cells.
- "Lymphoepithelial lesion" - gastric crypts invaded by a monomorphous population of lymphocytes.[38]
- Features:
- Cluster of lymphocytes - three cells or more - key feature.
- Single lymphocytes don't count.
- Clearing around the lymphocyte cluster.
- Cluster of lymphocytes - three cells or more - key feature.
- Associated with MALT lymphoma;[39] however, not specific.
- Features:
DDx:
IHC
- Panker -- most useful.
Others:
- CD3 (T cells) - scatter positivity.
- CD20 (B cells) +ve.
- CD138 (plasma cells).
- kappa, lambda -- often one is predominant, suggesting clonality.
- BCL2 +ve.
Treatment
- Triple therapy (two antibiotics, proton pump inhibitor (PPI)).[42]
- Surgery - if triple therapy fails.
Review paper: PMID 16950858.
Hereditary gastric cancer
Several syndromes are associated with gastric cancer:[43]
| Disease | Gene | Histology | Other |
|---|---|---|---|
| Hereditary diffuse gastric cancer (HDGC) syndrome | CDH1 (E-cadherin)[44] | diffuse - more specifically signet ring cell carcinoma | most important; assoc. invasive lobular carcinoma[45] |
| Lynch syndrome | MSH2, MLH1, others | ? | colorectal carcinoma, endometrial carcinoma |
| Familial adenomatous polyposis | APC | ? | adenomatous polyps |
| Peutz-Jeghers syndrome | STK11 | ? | stomach hamartomas - not precursor |
| Li-Fraumeni syndrome | TP53 (p53) | ? | AKA SBLA syndrome = sarcomas, breast, brain, leukemia, laryngeal, lung, adrenocortical carcinoma |
| Familial breast and ovarian cancer 2[46] | BRCA2 | ? | ? |
Gastric adenocarcinoma
General
Epidemiology:
- Prognosis is often poor as it is discovered at a late stage.
- Higher prevalence in countries in the far east (e.g. Japan) - thought to be environmental, e.g. diet.
Risk factors:
- Associated with helicobacter infections, i.e. Helicobacter gastritis.
- Alcohol - heavy use.[47]
- Genetic syndromes - see hereditary gastric cancer.
Note:
- Possible association with tobacco use - dependent on the study.[48]
Treatment:
- Surgical excision.
- Proximal tumours may require a complete gastrectomy as the stomach is innervated from its proximal part.
Classification
- Two different classification schemes.
- Lauren[49] - two types:
- Intestinal type (mass forming).
- Diffuse type (infiltrative).
- WHO classification - 6 subtypes for adenocarcinoma:[50]
- Papillary carcinoma.
- Tubular carcinoma.
- Mucinous carcinoma.
- Signet-ring carcinoma.
- Undifferentiated carcinoma.
- Adenosquamous carcinoma.
- Lauren[49] - two types:
Lame memory device STOMACH:
- Signet ring, Tubular, Oh papillary, Mucinous, Adenosquamouas, Crappy High grade (Undifferentiated).
Gross
Location:
- Large carcinomas preferentially involve the lesser curvature.[51]
- Ulceration with heaped (raised) edges.
- Appearance of the typical intestinal type tumour.
- Diffuse wall thickening with loss of the rugae - called linitis plastica.
- Typically due to diffuse carcinoma.
Main DDx of ulcer:
- Peptic ulcer disease - have a "punched-out" appearance: sharp edge, no granularity of surrounding mucosa.
Images:
- Linitis plastica - endoscopic image (WC).
- Ulcerating gastric carcinoma (WC).
- Ulcerating gastric carcinoma (WC).
Microscopic
Features - variable, either of the two following:
- "Typical adenocarcinoma":
- Gland-forming lesion that infiltrates into the lamina propria or beyond.
- Nuclear pleomorphism - common.
- +/-Signet ring carcinoma.
- Scattered single cells in the lamina propria or beyond with:
- Abundant cytoplasm containing one large (mucin-filled) vacuole.
- A peripheral nucleus (displaced by the vacuole).
- Scattered single cells in the lamina propria or beyond with:
DDx:
- Gastric xanthoma - may mimic signet ring cell carcinoma.
Images:
- WC:
- www:
Stains
- Mucicarmine +ve.
IHC
- CK7 +ve.
- CK20 -ve, occasionally +ve.
Others:
- p53 +ve in upto 75% of cases.[52]
Molecular
- May have HER2 over expression - more common in intestinal-type tumours.[53]
- Poor prognosis - like in breast cancer.
- Scoring system different than in breast cancer - complete membrane staining is not required.
Sign out
Biopsy
Intestinal type
STOMACH, BIOPSY: - INVASIVE ADENOCARCINOMA, INTESTINAL TYPE, MODERATELY DIFFERENTIATED. - Gastric mucosa with moderate chronic active inflammation and extensive intestinal metaplasia. - Benign small bowel mucosa with erosions.
Diffuse type
STOMACH, BIOPSY: - INVASIVE ADENOCARCINOMA, DIFFUSE TYPE. COMMENT: A pankeratin immunostain demonstrates single (infiltrating) epithelial cells in the lamina propria.
Micro
The tumour consists of single cells with abundant foamy-appearing cytoplasm and eccentric nuclei with mild nuclear atypia.
See also
References
- ↑ ALS. 4 Feb 2009.
- ↑ Rubio, CA. (Jun 2007). "Gastric duodenal metaplasia in duodenal adenomas.". J Clin Pathol 60 (6): 661-3. doi:10.1136/jcp.2006.039388. PMC 1955048. PMID 16837629. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1955048/.
- ↑ Osborn M, Mazzoleni G, Santini D, Marrano D, Martinelli G, Weber K (1988). "Villin, intestinal brush border hydrolases and keratin polypeptides in intestinal metaplasia and gastric cancer; an immunohistologic study emphasizing the different degrees of intestinal and gastric differentiation in signet ring cell carcinomas". Virchows Arch A Pathol Anat Histopathol 413 (4): 303–12. PMID 2459839.
- ↑ Braunstein, EM.; Qiao, XT.; Madison, B.; Pinson, K.; Dunbar, L.; Gumucio, DL. (May 2002). "Villin: A marker for development of the epithelial pyloric border.". Dev Dyn 224 (1): 90-102. doi:10.1002/dvdy.10091. PMID 11984877.
- ↑ Sternberg H4P 2nd Ed., P.484
- ↑ URL: http://www.lab.anhb.uwa.edu.au/mb140/CorePages/GIT/git.htm. Accessed on: 3 December 2010.
- ↑ http://www.histology-world.com/stains/stains.htm
- ↑ Goggin N, Rowland M, Imrie C, Walsh D, Clyne M, Drumm B (December 1998). "Effect of Helicobacter pylori eradication on the natural history of duodenal ulcer disease". Arch. Dis. Child. 79 (6): 502-5. PMC 1717771. PMID 10210995. http://adc.bmj.com/cgi/pmidlookup?view=long&pmid=10210995.
- ↑ http://www.histology-world.com/stains/stains.htm
- ↑ http://www.histology-world.com/stains/stains.htm
- ↑ Malfertheiner, P.; Chan, FK.; McColl, KE. (Oct 2009). "Peptic ulcer disease.". Lancet 374 (9699): 1449-61. doi:10.1016/S0140-6736(09)60938-7. PMID 19683340.
- ↑ Lin J, McKenna BJ, Appelman HD (November 2010). "Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: a controlled study". Am. J. Surg. Pathol. 34 (11): 1672–7. doi:10.1097/PAS.0b013e3181f3de93. PMID 20962621.
- ↑ RK. 13 December 2010.
- ↑ URL: http://www.springerlink.com/content/u2v2525241754557/ Accessed on: 19 November 2010.
- ↑ 15.0 15.1 Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 111. ISBN 978-0443066573.
- ↑ 16.0 16.1 Drude, RB.; Balart, LA.; Herrington, JP.; Beckman, EN.; Burns, TW. (Jun 1982). "Gastric xanthoma: histologic similarity to signet ring cell carcinoma.". J Clin Gastroenterol 4 (3): 217-21. PMID 6284833.
- ↑ Steen, S.; Lamont, J.; Petrey, L. (Jan 2008). "Acute gastric dilation and ischemia secondary to small bowel obstruction.". Proc (Bayl Univ Med Cent) 21 (1): 15-7. PMC 2190544. PMID 18209748. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2190544/.
- ↑ 18.0 18.1 Papanikolaou, IS.; Foukas, PG.; Sioulas, A.; Beintaris, I.; Panagopoulos, P.; Karamanolis, G.; Panayiotides, IG.; Dimitriadis, G. et al. (2011). "A case of gastric ischemic necrosis.". Endoscopy 43 Suppl 2 UCTN: E342. doi:10.1055/s-0030-1256795. PMID 22020717.
- ↑ Herman, J.; Chavalitdhamrong, D.; Jensen, DM.; Cortina, G.; Manuyakorn, A.; Jutabha, R. (Apr 2011). "The significance of gastric and duodenal histological ischemia reported on endoscopic biopsy.". Endoscopy 43 (4): 365-8. doi:10.1055/s-0030-1256040. PMID 21360426.
- ↑ Mesihovic, R.; Prohic, D.; Gribajcevic, M.; Vanis, N.; Gornjakovic, S.; Sarac, A. (2004). "Portal hypertensive gastropathy (PHG).". Med Arh 58 (6): 377-9. PMID 15648238.
- ↑ Thuluvath, PJ.; Yoo, HY. (Dec 2002). "Portal Hypertensive gastropathy.". Am J Gastroenterol 97 (12): 2973-8. doi:10.1111/j.1572-0241.2002.07094.x. PMID 12492178.
- ↑ 22.0 22.1 Sikanderkhel, S.; Luthra, M.; Chavalitdhamrong, D. (Jan 2012). "Snakeskin-like pattern mimicking portal hypertensive gastropathy in patient with eosinophilic gastritis.". Dig Endosc 24 (1): 53. doi:10.1111/j.1443-1661.2011.01155.x. PMID 22211417.
- ↑ 23.0 23.1 Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 120-1. ISBN 978-0443066573.
- ↑ Lam, MC.; Tha, S.; Owen, D.; Haque, M.; Chatur, N.; Gray, JR.; Yoshida, EM. (Nov 2011). "Gastric polyps in patients with portal hypertension.". Eur J Gastroenterol Hepatol 23 (12): 1245-9. doi:10.1097/MEG.0b013e32834c15cf. PMID 22002002.
- ↑ 25.0 25.1 Kamata, T.; Suzuki, H.; Yoshinaga, S.; Nonaka, S.; Fukagawa, T.; Katai, H.; Taniguchi, H.; Kushima, R. et al. (2012). "Localized gastric amyloidosis differentiated histologically from scirrhous gastric cancer using endoscopic mucosal resection: a case report.". J Med Case Rep 6 (1): 231. doi:10.1186/1752-1947-6-231. PMC 3438062. PMID 22863214. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3438062/.
- ↑ Wu, D.; Lou, JY.; Chen, J.; Fei, L.; Liu, GJ.; Shi, XY.; Lin, HT. (Nov 2003). "A case report of localized gastric amyloidosis.". World J Gastroenterol 9 (11): 2632-4. PMID 14606114.
- ↑ Sawada, T.; Adachi, Y.; Akino, K.; Arimura, Y.; Ishida, T.; Ishii, Y.; Endo, T. (2012). "Endoscopic features of primary amyloidosis of the stomach.". Endoscopy 44 Suppl 2 UCTN: E275-6. doi:10.1055/s-0032-1309750. PMID 22814919.
- ↑ 28.0 28.1 Jain, R.; Chetty, R. (Sep 2009). "Gastric hyperplastic polyps: a review.". Dig Dis Sci 54 (9): 1839-46. doi:10.1007/s10620-008-0572-8. PMID 19037727.
- ↑ URL: http://pathologyoutlines.com/stomach.html#hyperplastic. Accessed on: 26 July 2011.
- ↑ 30.0 30.1 Park, do Y.; Lauwers, GY. (Apr 2008). "Gastric polyps: classification and management.". Arch Pathol Lab Med 132 (4): 633-40. doi:10.1043/1543-2165(2008)132[633:GPCAM]2.0.CO;2. PMID 18384215. http://www.archivesofpathology.org/doi/full/10.1043/1543-2165(2008)132%5B633:GPCAM%5D2.0.CO;2.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 102. ISBN 978-0443066573.
- ↑ 32.0 32.1 Rugge, M.; Correa, P.; Dixon, MF.; Hattori, T.; Leandro, G.; Lewin, K.; Riddell, RH.; Sipponen, P. et al. (Feb 2000). "Gastric dysplasia: the Padova international classification.". Am J Surg Pathol 24 (2): 167-76. PMID 10680883.
- ↑ URL: http://surgpathcriteria.stanford.edu/gitumors/gastric-adenoma/printable.html. Accessed on: 18 December 2012.
- ↑ 34.0 34.1 Kushima, R.; Kim, KM. (Sep 2011). "Interobserver Variation in the Diagnosis of Gastric Epithelial Dysplasia and Carcinoma between Two Pathologists in Japan and Korea.". J Gastric Cancer 11 (3): 141-5. doi:10.5230/jgc.2011.11.3.141. PMID 22076218.
- ↑ Correa, P.; Piazuelo, MB. (Jan 2012). "The gastric precancerous cascade.". J Dig Dis 13 (1): 2-9. doi:10.1111/j.1751-2980.2011.00550.x. PMID 22188910.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/StomachNET_11protocol.pdf. Accessed on: 29 March 2012.
- ↑ Mbulaiteye, SM.; Hisada, M.; El-Omar, EM. (2009). "Helicobacter Pylori associated global gastric cancer burden.". Front Biosci 14: 1490-504. PMID 19273142.
- ↑ Bailey, D. 6 August 2010.
- ↑ Papadaki, L.; Wotherspoon, AC.; Isaacson, PG. (Nov 1992). "The lymphoepithelial lesion of gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT): an ultrastructural study.". Histopathology 21 (5): 415-21. PMID 1452124.
- ↑ Kim, K.; Kim, EJ.; Kim, MJ.; Song, HJ.; Lee, YS.; Jung, KW.; Yu, E. (Dec 2009). "Clinicopathological features of syphilitic gastritis in Korean patients.". Pathol Int 59 (12): 884-9. doi:10.1111/j.1440-1827.2009.02462.x. PMID 20021615.
- ↑ Long, BW.; Johnston, JH.; Wetzel, W.; Flowers, RH.; Haick, A. (Sep 1995). "Gastric syphilis: endoscopic and histological features mimicking lymphoma.". Am J Gastroenterol 90 (9): 1504-7. PMID 7661178.
- ↑ Zullo, A.; Hassan, C.; Andriani, A.; Cristofari, F.; De Francesco, V.; Ierardi, E.; Tomao, S.; Morini, S. et al. (Aug 2009). "Eradication therapy for Helicobacter pylori in patients with gastric MALT lymphoma: a pooled data analysis.". Am J Gastroenterol 104 (8): 1932-7; quiz 1938. doi:10.1038/ajg.2009.314. PMID 19532131.
- ↑ Sereno, M.; Aguayo, C.; Guillén Ponce, C.; Gómez-Raposo, C.; Zambrana, F.; Gómez-López, M.; Casado, E. (Sep 2011). "Gastric tumours in hereditary cancer syndromes: clinical features, molecular biology and strategies for prevention.". Clin Transl Oncol 13 (9): 599-610. PMID 21865131.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 192090
- ↑ Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R. et al. (Mar 1998). "E-cadherin germline mutations in familial gastric cancer.". Nature 392 (6674): 402-5. doi:10.1038/32918. PMID 9537325.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 600185
- ↑ Duell, EJ.; Travier, N.; Lujan-Barroso, L.; Clavel-Chapelon, F.; Boutron-Ruault, MC.; Morois, S.; Palli, D.; Krogh, V. et al. (Nov 2011). "Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.". Am J Clin Nutr 94 (5): 1266-75. doi:10.3945/ajcn.111.012351. PMID 21993435.
- ↑ Nomura, A.; Grove, JS.; Stemmermann, GN.; Severson, RK. (Nov 1990). "Cigarette smoking and stomach cancer.". Cancer Res 50 (21): 7084. PMID 2208177.
- ↑ LAUREN P (1965). "THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION". Acta Pathol Microbiol Scand 64: 31–49. PMID 14320675.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 823. ISBN 0-7216-0187-1.
- ↑ Yamagawa, H.; Onishi, T. (Sep 1989). "[A clinicopathological study of early gastric cancers with a diameter larger than five centimeters].". Gan No Rinsho 35 (10): 1114-8. PMID 2550682.
- ↑ Zali, MR.; Moaven, O.; Asadzadeh Aghdaee, H.; Ghafarzadegan, K.; Ahmadi, KJ.; Farzadnia, M.; Arabi, A.; Abbaszadegan, MR. (Jul 2009). "Clinicopathological significance of E-cadherin, β-catenin and p53 expression in gastric adenocarinoma.". J Res Med Sci 14 (4): 239-47. PMID 21772890.
- ↑ Romiti, A.; Di Rocco, R.; Milione, M.; Ruco, L.; Ziparo, V.; Zullo, A.; Duranti, E.; Sarcina, I. et al. (Jan 2012). "Somatostatin receptor subtype 2 A (SSTR2A) and HER2 expression in gastric adenocarcinoma.". Anticancer Res 32 (1): 115-9. PMID 22213295.