Difference between revisions of "Soft tissue lesions"
m (→Images) |
|||
| Line 709: | Line 709: | ||
<gallery> | <gallery> | ||
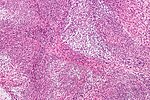
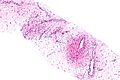
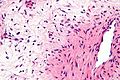
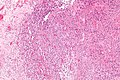
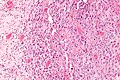
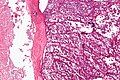
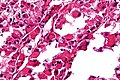
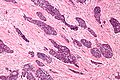
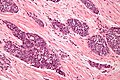
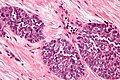
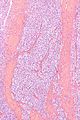
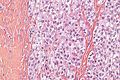
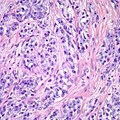
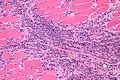
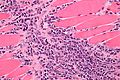
Image:Monophasic_synovial_sarcoma_-_intermed_mag.jpg | Monophasic synovial sarcoma with staghorn vessels - intermed. mag. (WC/Nephron) | Image:Monophasic_synovial_sarcoma_-_intermed_mag.jpg | Monophasic synovial sarcoma with staghorn vessels - intermed. mag. (WC/Nephron) | ||
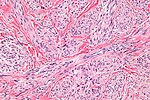
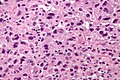
Image:Monophasic_synovial_sarcoma_-_high_mag.jpg | Monophasic synovial sarcoma with staghorn vessels - high mag. (WC/Nephron) | |||
</gallery> | </gallery> | ||
www: | www: | ||
Revision as of 20:41, 24 August 2013
Soft tissue lesions strike fear in many pathologists as they are uncommon and may be difficult to diagnose. Malignant soft tissue lesions, i.e. cancerous soft tissue lesions, are usually sarcomas. Sarcomas are malignancies derived from mesenchymal tissue.
Introduction
WHO classification of soft tissue lesions/tumours
Morphologic grouping
These include:[1]
- Adipocytic tumours.
- Fibroblastic/myofibroblastic tumours.
- "Fibrohistiocytic" tumours.
- Smooth muscle tumours.
- Skeletal muscle tumours.
- Vascular tumours.
- Perivascular (pericytic) tumours.
- Chondro-osseous tumours.
- Tumours of uncertain differentiation.
Biologic potential grouping
These include:[2]
- Benign.
- Intermediate (locally aggressive).
- Intermediate (rarely metastasizing).
- Malignant.
Prevalence
- All sarcomas are rare buggers.
- As the classification has been changing over the past years (with more subtypes being recognized/identified) numbers are variable from study-to-study.
- Once upon a time almost everything was called malignant fibrous histiocytoma; thus, it is listed as a common entity in some publications.
Most common:[3]
- Liposarcoma.
- Leiomyosarcoma.
Molecular testing
- Molecular testing plays an important role in soft tissue pathology.
- It is generally seen as an adjunct test that:[4]
- Often is used to confirm the histomorphologic impression/quality control.
- Frequently has some prognostic significance.
- May directly affect treatment.
Translocations
- Many tumours in soft tissue pathology are diagnosed inconjunction with the finding of chromosomal translocations.
Morphohistologic patterns
| Name | Description | DDx | Image(s) |
|---|---|---|---|
| Storiform, AKA patternless pattern[5] | whorled, cartwheel-like arrangement | pleomorphic undifferentiated sarcoma, solitary fibrous tumour, dermatofibrosarcoma protuberans, dermatofibroma[6] | |
| Herring bone | like herring bone (technique) for climbing a hill in cross country skiing; books on a shelf, where they have partially fallen over -- on the one shelf to the left and the one below to the right | fibrosarcoma, synovial sarcoma, MPNST | |
| Fasicular | the long axis of the (spindle) cells are perpendicular to one another in adjacent bundles of cells | leiomyoma, leiomyosarcoma | |
| Biphasic | nests of cells and stroma | synovial sarcoma, DSRCT, alveolar RMS |
Notes:
- Memory device: herring bone DDx MSF = MPNST, Synovial sarcoma, Fibrosarcoma.
Grading
- Several systems exist.
- The US-CAP advocates the use of the French system over the NCI system.
- The French system is a better predictor metastases and mortality.[7]
French system
- Formally known as the grading system from the French Federation of Cancer Centres Sarcoma Group (FNCLCC).
Overview
- Differentiation (score 1-3).
- De facto, this is mostly the histologic type.
- Mitotic rate (score 1-3).
- Necrosis (score 0-2)
Obtaining a score:
- Add all the points from the three components.
Scoring:
- Grade 1 = 2-3.
- Grade 2 = 4-5.
- Grade 3 = 6-8.
Differentiation
- Standardized for histologic types.
- Most tumours = 3/3.
Exceptions:[8]
- Well-differentiated liposarcoma = 1.
- Myxoid liposarcoma = 2.
- Conventional liposarcoma = 2.
- Fibrosarcoma = 2.
- Myxofibrosarcoma =2.
A group of tumours is not graded:[8]
- MPNST.
- Rhabdomyosarcoma.
- Alveolar soft part sarcoma.
- Clear cell sarcoma.
- Extraskeletal myxoid chondrosarcoma.
Mitotic rate
- 0-9 mitoses/10 HPF.
- 10-19 mitoses/10 HPF.
- >=20 mitoses/10 HPF.
Notes:
- 1 HPF = 0.1734 mm^2.
- Most resident microscopes have a field of view = 0.2376 mm^2.
- Thus, ~7.3 HPFs on a resident microscope corresponds to 10 US-CAP HPFs.
- Most resident microscopes have a field of view = 0.2376 mm^2.
Necrosis
- None = score 0.
- <=50% of tumour = score 1.
- >50% of tumour = score 2.
System used by some at MSH
Some pathologists at MSH use the system advocated by Costa et al..[9]
Scoring
- Grade 1 = 1 point.
- Grade 2 = 2 points.
- Grade 3 = 3-4 points.
Components
Points for each of the following:
- Mitotic activity >= 6 / 10 HPF @ 40X - definition suffers from HPFitis.
- Pleomorphism present.
- Cellularity (cells/matrix) > 50%.
- Necrosis >15% - microscopic (without targeting necrosis grossly) or grossly.
Stage
Lymph node metastases in sarcomas
- Lymph node (LN) spread is uncommon in sarcomas; lymph node metastases are seen in <3% of cases.[10]
- Many sarcomas are reported in LNs.
- According to the CAP checklist for soft tissue[11] the most common are: epithelioid sarcoma and clear cell sarcoma.
- According to Fong et al.[10] the most commonly is: angiosarcoma.
- Many sarcomas are reported in LNs.
Sarcomas more likely to be found in the lymph nodes - mnemonic RACE For MS:[12]
- Rhabdomyosarcoma
- Angiosarcoma.
- Clear cell sarcoma.
- Epitheliod sarcoma.
- Fibrosarcoma.
- Malignant fibrous histiocytoma (pleomorphic undifferentiated sarcoma.
- Synovial cell sarcoma.
DDx by history/site
Retroperiteum
Note: Synovial sarcoma and fibrosarcoma are very rare in the retroperitoneum.
Young person - extremity sarcoma
Gross characteristics
- Usually non-specific.
- Most sarcomas have a pushing border.
- If there is an infiltrative border think: (1) fibromatosis, (2) carcinoma.
Adipocytic tumours
This category includes:
- Lipoma.
- Liposarcoma.
- Hibernoma.
Smooth muscle tumours
IHC markers: desmin, SMA, H-caldemsin (most specific).
Leiomyosarcoma
Microscopic
Features (summary):
- Fasicular cellular spindle cell lesion with:
- Nuclear atypia.
- Necrosis.
- High mitotic rate.
Fibrohistiocytic tumours
Fibrohistiocytic refers (only) to the histomorphologic appearance and therefore may be written in quotation marks; these tumours are not derived from histiocytes (or tissue macrophages), as the name implies.[13]
Pleomorphic undifferentiated sarcoma
- Abbreviated PUS.
- AKA Undifferentiated pleomorphic sarcoma, abbreviated UPS.
- Previously known as malignant fibrous histiocytoma, abbreviated MFH.[14]
General
- Common sarcoma.
- Usually deep tissue of the trunk and extremities.
- A diagnosis of exclusion[15] / wastebasket for unclassifiable high grade sarcomas.
Microscopic
Features:[16]
- Storiform pattern (AKA patternless pattern) - key feature.
- Marked nuclear pleomorphism key feature.
- Variation is nuclear size, nuclear shape and nuclear staining (esp. hyperchromasia).
- Mitoses - abundant; atypical mitoses common.
- Necrosis (common).
- Mix of spindle cells and epithelioid cells.
- Deep to skin - important.
Other findings:
- +/-Giant cells (see subclassification).
- +/-Inflammation (see subclassification).
- Neutrophils.
- Eosinophils.
Notes:
- Superficial lesions with the morphology of PUS are called by some atypical fibroxanthomas (AFXs).
DDx:
- Atypical fibroxanthoma (AFX) - superficial skin.
- Dedifferentiated liposarcoma.
- Leiomyosarcoma.
- Metaplastic carcinoma.
- Malignant melanoma.
- Rhabdomyosarcoma.
- Synovial sarcoma.
- Others.
Images
Subclassification
Pleomorphic sarcoma (PS) is subclassified the following way:[17]
- PS with giant cells.
- PS with inflammation.
- PUS (not otherwise specified) - wastebasket diagnosis; if neither of the above two apply.
IHC
Exclusionary stains - should be negative:
- AE1/AE3.
- p63.
- Myogenin.
- S-100.
- HMB-45.
Usually negative, may be positive:[18]
- Desmin.
- SMA.
Commonly positive:
- CD68.[18]
- Vimentin.
Fibroblastic/myofibroblastic tumours
This is a very large and important group of soft tissue lesions. It is covered in a separate article.
The grouping includes:
- Inflammatory myofibroblastic tumour.
- Nodular fasciitis.
- Desmoid-type fibromatosis (Desmoid tumour).
- Proliferative fasciitis.
- Solitary fibrous tumour (Hemangiopericytoma).
- Desmoplastic fibroblastoma.
- Low-grade fibromyxoid sarcoma.
- Others.
Perivascular tumours
This grouping includes only two:[19]
- Glomus tumour - both benign and malignant.
- Myopericytoma.
Vascular lesions
Vascular lesions are "too red"; they have too many RBCs.
They include:
Skeletal muscle tumours
Rhabdomyoma
Rhabdomyosarcoma
- Abbreviated RMS.
Comes it two main flavours:
- Alveolar rhabdomyosarcoma.
- Embryonal rhabdomyosarcoma.
The histology may be that of a small round cell tumour.
Chondro-osseous tumours
This grouping includes tumours derived from cartilage and bone.
Tumours of uncertain differentiation
Angiomatoid fibrous histiocytoma
General
- Rarely metastasizes.
- Children & young adults.
Microscopic
Features:[20]
- Cystic spaces with blood - simulates a vascular neoplasm.[21]
- Epithelioid to spindle cells.
- May have a histiocytic appearance.[22]
- Inflammation.
- Lymphoid cuff[23] - lymphocytes around periphery of lesion.
- Hemorrhage.
Note:
- The first impression may be that it is granulomatous inflammation; however, the cytoplasm doesn't fit (it isn't bubbly and it isn't sheet-like), and the nuclei aren't quite right (few footprint shaped nuclei).
Images
www:
IHC
Features:[20]
- CD68 +ve.
- CD57 +ve.
- Desmin +ve.
- Vimentin +ve.
Molecular
AFH has recurrent translocations:
- t(12;16) FUS/ATF1.
- t(12;22) EWS/ATF1.
Aggressive angiomyxoma
- AKA deep aggressive angiomyxoma.
General
- Classically a vulvar mass or (less commonly) a scrotal mass.
- Case report of a thigh lesion.[24]
- Benign - no metastatic potential.
- "Aggressive" as it has a high recurrance.
Gross
- Poorly circumscribed.
- "Rubbery" or "gelatinous".
Location:
- Vulva - classic location.
Clinical DDx:
Microscopic
Features:[25]
- Thick blood vessels that meld into the surrounding stroma - key feature.
- Myxoid stroma - key feature.
- Small stellate cell/spindle cells without significant nuclear atypia.
DDx:
Images
www:
- Aggressive angiomyxoma - low mag. (webpathology.com).
- Aggressive angiomyxoma - high mag. (webpathology.com).
- Aggressive angiomyxoma - several images (upmc.edu).
IHC
Features:[25]
- Desmin +ve.
- Vimentin +ve.
- ER +ve.
- PR +ve.
Angiomyofibroblastoma
General
- Uncommon.
Clinical DDx:
Microscopic
Features:[26]
- Hypercellular zones and hypocellular edematous zones.
- Small blood vessels (~20 micrometers) - no large blood vessels - key feature.
- Myxoid stroma - key feature.
- Small stellate cell/spindle cells without significant nuclear atypia.
DDx:
- Aggressive angiomyxoma - less cellular, large blood vessels.
Images:
Extrarenal malignant rhabdoid tumour
- Essentially identical to renal malignant rhabdoid tumour.[27]
General
- Usu. children < 2 years old.
- Very poor prognosis.
- In the CNS it is known as atypical teratoid-rhabdoid tumour.[27]
Microscopic
Features:[27]
- Variable architecture.
- Round cells.
- Eccentric vesicular nucleus.
- Prominent nucleolus -- key feature.
IHC
- INI1 (SMARCB1) -ve.
- AKA BAF47.
Ewing sarcoma/PNET
- A small round blue cell tumour that may be seen in bone. It is discussed in the context of bone tumours.
Epithelioid sarcoma
- Sarcomas with an epithelioid morphology are covered in epithelioid sarcomas.
General
- Rare.
- Adolescents, young adults.
- Serum CA-125 may be useful for following clinically.[28]
Subclassification:[29]
- Proximal type:
- More aggressive.
- Distal type:
Microscopic
Features:[30]
- Epithelioid morphology and spindle morphology - which predominates is dependent on location (see subclassification).
- +/-Prominent nucleolus - distinctive feature.
- Zonal necrosis with irregular border.
- Descriptors: "Garland necrosis", necrosis with "scalloped border" = necrotic regions with irregular border.
Subclassification:[29]
- Proximal-type (proximal location):
- More epithelioid.
- Distal-type (distal location):
- More spindled.
- Granuloma-like pattern.
DDx:
- Carcinoma.
- Rheumatoid nodule.
- Granuloma annulare.
Images
www:
IHC
Features:[31]
- INI1 (SMARCB1[32]) -ve.[33]
- Vimentin +ve.
- Various keratins +ve.
- Keratin 8, Keratin 19 +ve.
- 34betaE12 +ve/-ve.
- CD34 +ve.
- Malignant rhabdoid tumour -ve.
Others:
Alveolar soft part sarcoma
- Abbreviated ASPS.
General
- Adolescents/young adults.
- Children -- classically location: base of tongue and orbit.
- Typical indolent initially - ultimately a poor prognosis.[36]
Microscopic
Features:[27]
- Arranged in nest/separated by thin septa; vaguely resembles alveoli (at low power).
- Large cells (~30-50 μm) with abundant eosinophilic cytoplasm.
- An eccentric nucleus.
- +/-Nucleolus.
DDx:
- Rhabdomyosarcoma, alveolar - usu. does not have sheets of rhabdoid cells.
Images
www:
Stains
- PAS +ve (cytoplasmic) - considered the most useful.[37]
- PASD +ve (cytoplasmic).
IHC
- TFE3 +ve -- suggestive of characteristic translocation.
Molecular
- t(X;17)(p11.2;q25).[38]
Note:
- Same translocation may be seen in renal tumour with Xp11.2 translocation.
EM
- Distinctive membrane-bound intracytoplasmic crystal lattice with ~5 nm fibres spaced 10 nm apart.[36]
Image:
Desmoplastic small round cell tumour
- Abbreviated DSRCT.
General
- Males > females.
- Usu. affects young adults.
- Typically retroperitoneal.
- Poor prognosis.
Microscopic
Features:[39]
- Broad bands of paucicellular fibrous stroma with:
- Small round cells in nests with an undulating sharp border.
- The small round cells lack distinct nucleoli and have scant cytoplasm; they are small round cell tumour cells.
Notes:
- Usu. abundant mitoses.
- +/-Necrosis.
DDx:
- Metastatic germ cell tumour (DDx of location and age).
- Embryonal RMS.
- It should be noted that DSRCT, like embryonal RMS, is +ve for desmin!
- Solid variant of alveolar RMS.
- Nests in alveolar RMS have round edges.
Images
IHC
Features:
- AE1/AE3 +ve.
- Desmin +ve.
- EMA +ve.
- Actin -ve.
- WT1 (N-terminal) -ve.
- WT1 (C-terminal) +ve.
- CD57 +ve.
Molecular
Clear cell sarcoma
- Known among pathologists as "soft-tissue melanoma" and "melanoma of the soft parts", as it has a strong morphological resemblance.[42]
- Not to be confused with clear cell sarcoma of the kidney.
General
- Molecular changes and origin distinct from malignant melanoma.
- Incidence: rare soft tissue tumour.
Clinical
- Usually - deep soft tissue or extremities.
- Classically associated with tendons and aponeuroses.[43]
- Guarded prognosis.
- First described in 1965.[44]
Microscopic
Features:[42]
- Architecture: sheeting or fascicular (bundles) arrangement.
- Fibrous septae - between tumour cells.
- Tumour cells uniform (low pleomorphism) - spindle-shaped or epithelioid:
- Classically have clear cytoplasm.
- Prominent nucleoli - basophilic - key feature.
- +/-Binucleation.
DDx:
- Malignant melanoma.
- PEComa.
- Carcinoma.
Images
IHC
Features:[42]
- S100 +ve.
- HMB-45 +ve.
- Melan A (MART-1) +ve; sometimes -ve.
- BCL2 +ve.
- CD57 +ve (usually).
Keratins:
- EMA may be +ve.
- CAM5.2 -ve.
- AE1/AE3 -ve.
Molecular studies
- Chromosomal translocation t(12;22)(q13;q12).[42]
- Fusion transcripts:
- EWSR1-ATF1.
- Same translocation in: myxoid liposarcoma,[45] and hyalinizing clear cell carcinoma.
- EWSR1-CREB1 (GI tract associated).
- EWSR1-ATF1.
- Fusion transcripts:
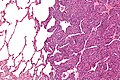
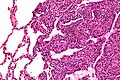
Synovial sarcoma
- Abbreviated SS.
General
- Does not arise from cartilage.[27]
- Young adults or adolescents.
- Classic age: 30s.
- Poor prognosis.
Clinical:[46]
- Present with soft palpable mass - slow growing - often for years.
- May present with pain.
- Uncommon finding in sarcomas.
Gross
Location:
- Usually close to a joint.
- Usually distal extremity ~65% of cases.[46]
- Upper extremity ~20% of cases.[46]
Appearance - often non-specific:
- Solid often lobulated +/- cystic component.
- Grey-yellow.
- Pushing border to ill-defined border.
Images:
Microscopic
Comes in three (histologic) flavours:[27][48]
- Spindle cell sarcoma with features of hemangiopericytoma, i.e. staghorn vessels.
- Biphasic synovial sarcoma:
- Spindle cells with features of hemangiopericytoma.
- Epitheliod glands or nests.
- Primitive round cell type.
Features:
- Herring bone or vesicular pattern - key feature.
- Spindle cells.
- +/-Glandular component - typically more pink.
- +/-Calcification - uncommon.
- Extensive calcification = better prognosis.[49]
DDx:
- MPNST.
- Can be difficult.
Images
www:
- Synovial sarcoma (scielo.br).
- Synovial sarcoma - collection of images (humpath.com).
- Synovial sarcoma - several images (upmc.edu).
- Biphasic SS (radiographics.rsna.org).
- Monophasic SS (radiographics.rsna.org).
IHC
Features:[27]
- Vimentin +ve.
- EMA +ve.
- BCL2 +ve.
- CD99 +ve.
Others:
- Beta-catenin +ve ~30-70%.[50]
- Cyclin D1 ~50%.[50][51]
- TLE1 +ve nuclear staining; not specific for synovial sarcoma.[52][53]
Typically negative:[54]
- CD34.
- S100 ~30% +ve.
- SMA.
Notes:
- Synovial sarcoma & MPNST:
- Both +ve: PGP9.5 (UCHL1[55]), S100, NGFR, CD56, CD99, vimentin.
- Synovial +ve: EMA, keratin, BCL2, TLE1.
- MPNST +ve: nestin, CD34.
Trivia:
- PGP in PGP9.5 = protein gene product.[56]
EM
Features:[57]
- Biphasic tumour have biphasic ultrastructural features (unlike spindle cell carcinoma and epithelioid sarcoma).
- Epithelioid component is adenocarcinoma-like - they have:
- Intermediate filaments.
- Tonofilaments.
- Microvilli.
- Spindle cell component - mostly features less.
- Poorly formed desmosomes.
- No intermediate filaments, no myofilaments.
Molecular pathology
Associated translocation:
- t(X;18)(p11.2;q11.2).[58]
- SYT/SSX fusion gene.
Several SSX genes - cannot be differentiated with standard karyotyping:
- SSX1.
- SSX2 - better survival, rarely seen in biphasic tumours.[59]
- SSX4 - uncommon.
Notes:
- At HSC t(X,18) = synovial sarcoma.
Other
Granulocytic sarcoma
- Common alternate terms: myeloid sarcoma, chloroma.
- Other terms:[60] myeloblastoma, chloromyeloma, chloromyelosarcoma, granulocytic leukosarcoma, or myelosarcoma.
General
- Soft tissue manifestation of acute myeloid leukemia.[60]
Microscopic
Features:
- Cluster of atypical small blue cells in soft tissue.
Note:
- May mimic small cell carcinoma, large cell lymphomas (DLBCL, ALCL), small round cell tumours.
Images
www:
- Granulocytic sarcoma - several crappy images (upmc.edu).
- Myeloid sarcoma - several images (upmc.edu).
See also
- Bone.
- Dermatopathology.
- Hematopathology.
- Spindle cell lesion.
- Neurofibromatosis.
- Small round cell tumours.
References
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 601-3. ISBN 978-0781765275.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 598-604. ISBN 978-0781765275.
- ↑ Skubitz KM, D'Adamo DR (November 2007). "Sarcoma". Mayo Clin. Proc. 82 (11): 1409–32. PMID 17976362. http://www.mayoclinicproceedings.com/content/82/11/1409.long.
- ↑ Fletcher CD, Fletcher JA, Dal Cin P, Ladanyi M, Woodruff JM (July 2001). "Diagnostic gold standard for soft tissue tumours: morphology or molecular genetics?". Histopathology 39 (1): 100–3. PMID 11454050.
- ↑ Mangano WE, Cagle PT, Churg A, Vollmer RT, Roggli VL (August 1998). "The diagnosis of desmoplastic malignant mesothelioma and its distinction from fibrous pleurisy: a histologic and immunohistochemical analysis of 31 cases including p53 immunostaining". Am. J. Clin. Pathol. 110 (2): 191–9. PMID 9704618.
- ↑ Meister P, Höhne N, Konrad E, Eder M (July 1979). "Fibrous histiocytoma: an analysis of the storiform pattern". Virchows Arch A Pathol Anat Histol 383 (1): 31–41. PMID 224569.
- ↑ 7.0 7.1 Guillou L, Coindre JM, Bonichon F, et al. (January 1997). "Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma". J. Clin. Oncol. 15 (1): 350–62. PMID 8996162.
- ↑ 8.0 8.1 8.2 URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/SoftTissue_11protocol.pdf. Accessed on: 12 April 2011.
- ↑ Costa J, Wesley RA, Glatstein E, Rosenberg SA (February 1984). "The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases". Cancer 53 (3): 530–41. PMID 6692258.
- ↑ 10.0 10.1 Fong, Y.; Coit, DG.; Woodruff, JM.; Brennan, MF. (Jan 1993). "Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients.". Ann Surg 217 (1): 72-7. PMC 1242736. PMID 8424704. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1242736/.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/SoftTissue_11protocol.pdf. Accessed on: 28 March 2012.
- ↑ URL: http://www.aippg.net/forum/f21/surgery-mnemonics-79897/. Accessed on: 23 March 2012.
- ↑ Luzar, B.; Calonje, E. (Jan 2010). "Cutaneous fibrohistiocytic tumours - an update.". Histopathology 56 (1): 148-65. doi:10.1111/j.1365-2559.2009.03447.x. PMID 20055912.
- ↑ URL: http://sarcomahelp.org/learning_center/mfh.html. Accessed on: 8 April 2011.
- ↑ Matushansky I, Charytonowicz E, Mills J, Siddiqi S, Hricik T, Cordon-Cardo C (August 2009). "MFH classification: differentiating undifferentiated pleomorphic sarcoma in the 21st Century". Expert Rev Anticancer Ther 9 (8): 1135–44. doi:10.1586/era.09.76. PMC 3000413. PMID 19671033. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3000413/.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 613. ISBN 978-0781765275.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 613-4. ISBN 978-0781765275.
- ↑ 18.0 18.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 613. ISBN 978-0781765275.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 602. ISBN 978-0781765275.
- ↑ 20.0 20.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 624-5. ISBN 978-0781765275.
- ↑ Enzinger, FM. (Dec 1979). "Angiomatoid malignant fibrous histiocytoma: a distinct fibrohistiocytic tumor of children and young adults simulating a vascular neoplasm.". Cancer 44 (6): 2147-57. PMID 228836.
- ↑ URL: http://dermatology.cdlib.org/1605/1_case_reports/4_09-00041/patrizi.html. Accessed on: 15 November 2011.
- ↑ 23.0 23.1 Matsumura, T.; Yamaguchi, T.; Tochigi, N.; Wada, T.; Yamashita, T.; Hasegawa, T. (Feb 2010). "Angiomatoid fibrous histiocytoma including cases with pleomorphic features analysed by fluorescence in situ hybridisation.". J Clin Pathol 63 (2): 124-8. doi:10.1136/jcp.2009.072256. PMID 20154033. http://jcp.bmj.com/content/63/2/124.full.
- ↑ Heffernan, EJ.; Hayes, MM.; Alkubaidan, FO.; Clarkson, PW.; Munk, PL. (Jul 2008). "Aggressive angiomyxoma of the thigh.". Skeletal Radiol 37 (7): 673-8. doi:10.1007/s00256-008-0465-0. PMID 18338163.
- ↑ 25.0 25.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 624. ISBN 978-0781765275.
- ↑ 26.0 26.1 Fletcher, CD.; Tsang, WY.; Fisher, C.; Lee, KC.; Chan, JK. (Apr 1992). "Angiomyofibroblastoma of the vulva. A benign neoplasm distinct from aggressive angiomyxoma.". Am J Surg Pathol 16 (4): 373-82. PMID 1314521.
- ↑ 27.0 27.1 27.2 27.3 27.4 27.5 27.6 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 627. ISBN 978-0781765275.
- ↑ 28.0 28.1 Hoshino, M.; Kawashima, H.; Ogose, A.; Kudo, N.; Ariizumi, T.; Hotta, T.; Umezu, H.; Hatano, H. et al. (Mar 2010). "Serum CA 125 expression as a tumor marker for diagnosis and monitoring the clinical course of epithelioid sarcoma.". J Cancer Res Clin Oncol 136 (3): 457-64. doi:10.1007/s00432-009-0678-1. PMID 19756736.
- ↑ 29.0 29.1 Guillou L, Wadden C, Coindre JM, Krausz T, Fletcher CD (February 1997). ""Proximal-type" epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. Clinicopathologic, immunohistochemical, and ultrastructural study of a series". Am. J. Surg. Pathol. 21 (2): 130–46. PMID 9042279.
- ↑ The International Agency for Research on Cancer (Editors: Fletcher, C.D.M.; Unni, K. Krishnan; Mertens, F.) (2006). Pathology and Genetics of Tumours of Soft Tissue and Bone (IARC WHO Classification of Tumours) (3rd ed.). World Health Organization. pp. 205. ISBN 978-9283224136.
- ↑ Miettinen M, Fanburg-Smith JC, Virolainen M, Shmookler BM, Fetsch JF (August 1999). "Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis". Hum. Pathol. 30 (8): 934–42. PMID 10452506.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 601607
- ↑ Mentzel T (March 2010). "[Epithelioid sarcoma: morphologic variants and differential diagnosis]" (in German). Pathologe 31 (2): 135–41. doi:10.1007/s00292-009-1250-0. PMID 19997734.
- ↑ Humble, SD.; Prieto, VG.; Horenstein, MG. (Apr 2003). "Cytokeratin 7 and 20 expression in epithelioid sarcoma.". J Cutan Pathol 30 (4): 242-6. PMID 12680954.
- ↑ Lee, HI.; Kang, KH.; Cho, YM.; Lee, OJ.; Ro, JY. (Jun 2006). "Proximal-type epithelioid sarcoma with elevated serum CA 125: report of a case with CA 125 immunoreactivity.". Arch Pathol Lab Med 130 (6): 871-4. doi:10.1043/1543-2165(2006)130[871:PESWES]2.0.CO;2. PMID 16740043.
- ↑ 36.0 36.1 36.2 Folpe, AL.; Deyrup, AT. (Nov 2006). "Alveolar soft-part sarcoma: a review and update.". J Clin Pathol 59 (11): 1127-32. doi:10.1136/jcp.2005.031120. PMC 1860509. PMID 17071801. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860509/.
- ↑ Zarrin-Khameh, N.; Kaye, KS. (Mar 2007). "Alveolar soft part sarcoma.". Arch Pathol Lab Med 131 (3): 488-91. doi:10.1043/1543-2165(2007)131[488:ASPS]2.0.CO;2. PMID 17516754.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 606243
- ↑ Pickhardt PJ, Fisher AJ, Balfe DM, Dehner LP, Huettner PC (March 1999). "Desmoplastic small round cell tumor of the abdomen: radiologic-histopathologic correlation". Radiology 210 (3): 633–8. PMID 10207460. http://radiology.rsna.org/content/210/3/633.full.
- ↑ Lee YS, Hsiao CH (October 2007). "Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical and molecular study of four patients". J. Formos. Med. Assoc. 106 (10): 854–60. doi:10.1016/S0929-6646(08)60051-0. PMID 17964965.
- ↑ Lal DR, Su WT, Wolden SL, Loh KC, Modak S, La Quaglia MP (January 2005). "Results of multimodal treatment for desmoplastic small round cell tumors". J. Pediatr. Surg. 40 (1): 251–5. doi:10.1016/j.jpedsurg.2004.09.046. PMID 15868593. http://www.dsrct.com/JPS%20Article.pdf.
- ↑ 42.0 42.1 42.2 42.3 Hisaoka M, Ishida T, Kuo TT, et al. (March 2008). "Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases". Am. J. Surg. Pathol. 32 (3): 452–60. doi:10.1097/PAS.0b013e31814b18fb. PMID 18300804.
- ↑ Dim, DC.; Cooley, LD.; Miranda, RN. (Jan 2007). "Clear cell sarcoma of tendons and aponeuroses: a review.". Arch Pathol Lab Med 131 (1): 152-6. doi:10.1043/1543-2165(2007)131[152:CCSOTA]2.0.CO;2. PMID 17227118.
- ↑ URL: http://www.informaworld.com/smpp/723576818-750600/ftinterface~db=all~content=a789166263~fulltext=713240928. Accessed on: 5 May 2010.
- ↑ Suzuki, K.; Matsui, Y.; Endo, K.; Kubo, T.; Hasegawa, T.; Kimura, T.; Ohtani, O.; Yasui, N. (Nov 2010). "Myxoid liposarcoma with EWS-CHOP type 1 fusion gene.". Anticancer Res 30 (11): 4679-83. PMID 21115923.
- ↑ 46.0 46.1 46.2 Murphey, MD.; Gibson, MS.; Jennings, BT.; Crespo-Rodríguez, AM.; Fanburg-Smith, J.; Gajewski, DA.. "From the archives of the AFIP: Imaging of synovial sarcoma with radiologic-pathologic correlation.". Radiographics 26 (5): 1543-65. doi:10.1148/rg.265065084. PMID 16973781.
- ↑ URL: http://www.sarcomaimages.com/index.php?v=Synovial-Sarcoma. Accessed on: 2 April 2012.
- ↑ Schaal CH, Navarro FC, Moraes Neto FA (2004). "Primary renal sarcoma with morphologic and immunohistochemical aspects compatible with synovial sarcoma". Int Braz J Urol 30 (3): 210–3. PMID 15689250. http://www.brazjurol.com.br/may_june_2004/Schaal_ing_210_213.htm.
- ↑ Varela-Duran, J.; Enzinger, FM. (Jul 1982). "Calcifying synovial sarcoma.". Cancer 50 (2): 345-52. PMID 6282441.
- ↑ 50.0 50.1 Horvai AE, Kramer MJ, O'Donnell R (June 2006). "Beta-catenin nuclear expression correlates with cyclin D1 expression in primary and metastatic synovial sarcoma: a tissue microarray study". Arch. Pathol. Lab. Med. 130 (6): 792–8. PMID 16740029.
- ↑ Ng TL, Gown AM, Barry TS, et al. (January 2005). "Nuclear beta-catenin in mesenchymal tumors". Mod. Pathol. 18 (1): 68–74. doi:10.1038/modpathol.3800272. PMID 15375433.
- ↑ Kosemehmetoglu K, Vrana JA, Folpe AL (July 2009). "TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms". Mod. Pathol. 22 (7): 872–8. doi:10.1038/modpathol.2009.47. PMID 19363472. http://www.nature.com/modpathol/journal/v22/n7/full/modpathol200947a.html.
- ↑ Seo SW, Lee H, Lee HI, Kim HS (February 2011). "The role of TLE1 in synovial sarcoma". J Orthop Res. doi:10.1002/jor.21318. PMID 21319215.
- ↑ URL: http://path.upmc.edu/cases/case292/dx.html. Accessed on: 14 January 2012.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 191342
- ↑ Doran, JF.; Jackson, P.; Kynoch, PA.; Thompson, RJ. (Jun 1983). "Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis.". J Neurochem 40 (6): 1542-7. PMID 6343558.
- ↑ Fisher, C. (Dec 1998). "Synovial sarcoma.". Ann Diagn Pathol 2 (6): 401-21. PMID 9930576.
- ↑ URL: http://www.ncbi.nlm.nih.gov/omim/300813. Accessed on: 30 May 2010.
- ↑ Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 625 Q6. ISBN 978-1416025887.
- ↑ 60.0 60.1 Eom, KS.; Kim, TY. (Mar 2011). "Intraparenchymal myeloid sarcoma and subsequent spinal myeloid sarcoma for acute myeloblastic leukemia.". J Korean Neurosurg Soc 49 (3): 171-4. doi:10.3340/jkns.2011.49.3.171. PMC 3085814. PMID 21556238. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3085814/.