Lung tumours
Lung tumours comes to pathology to get diagnosed. This article basically deals with core biopsies. Pulmonary cytopathology is dealt with in the pulmonary cytopathology article.
An introduction to lung pathology is found in the pulmonary pathology article.
Lung tumours overview
Schematic overview of lung cancer (clinical)
| Lung cancer | |||||||||||||||||||||||||||||||||||||||||
| Primary | Metastatic | ||||||||||||||||||||||||||||||||||||||||
| NSCLC | SCLC | ||||||||||||||||||||||||||||||||||||||||
- NSCLC = non-small cell lung cancer.
- SCLS = small cell lung cancer.
Basic pathologic approach to lung cancer
| Lung cancer | |||||||||||||||||||||||||||||||
| Adenocarcinoma | Squamous cell carcinoma | SCLC | LCLC | ||||||||||||||||||||||||||||
- LCLC = large cell lung cancer.
- SCLS = small cell lung cancer.
Notes:
- Most lung cancer fits into one of the above categories.
- All types may be metastatic. Pathologists usually don't have to sort this out, as the clinican often knows whether a given lesion is metastatic (when correlated with radiology).
- Lung cancers may have a mixed morphology, e.g. SCLS may have squamous component.[1]
- Categorization as non-small cell lung cancer (NSCLC) should be avoided, as treatment is now somewhat dependent on subcategorization.[2]
Major types (primary)
Mnemonic ASSL:
- Adenocarcinoma.
- Squamous cell carcinoma.
- Small cell carcinoma (SCLC).
- Large cell carcinoma (LCLC).
Epidemiology
- Adenocarcinoma is the most common (primary lung cancer).[3]
- Adenocarcinoma is the non-smoker tumour - SCLC and squamous are more strongly associated with smoking.
Distribution
- Distribution - think about the location of letters in mnemonic ASSL.
- Adenocarcinoma is usually periperal, i.e. smaller airways.
- Squamous cell carcinoma and small cell carcinoma are typically central.
Management of primary lung cancer
Management is currently determined by categorization into:
- Small cell cancer.
- Non-small cell cancer (includes adenocarcinoma, squamous cell carcinoma, large cell carcinoma).
Microscopic features overview
Adenocarcinoma
- Glands or cytoplasm with mucin.
Squamous cell carcinoma
- Distinct cell borders with intercellular bridges.
- Eosinophilic cytoplasm.
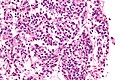
Small cell carcinoma
- Very cellular.
- Large NC ratio - very small amount of cytoplasm.
- Cells fragile - they tend to look "smudged" (Azzopardi phenomenon).
IHC
There is a great review paper by Jagirdar.[4]
Small cell carcinoma
- CD56 +ve - sensitive.[5]
- CK7 -ve, CK20 -ve.
Note:
- CD56 - cytoplasmic.[6]
Squamous cell carcinoma
- CK7 -ve, CK20 -ve.
- HMWK +ve.
- Usually TTF-1 -ve.[7]
- p40 +ve.
Primary vs. secondary
- TTF-1 is considered useful.[4]
- 75% +ve adenocarcinoma
- 11% +ve SSC
- 50% +ve large cell carcinoma
- 0% +ve mesothelioma
- significant rates of +ve in some metastatic tumours -- see article by Jagirdar.
Note:
- TTF-1 - should be nuclear staining; cytoplasmic staining is non-specific.[8]
Neuroendocrine tumours
Overview
- This is a group of tumours that has benign (e.g. carcinoid tumour of the lung) to malignant (e.g. small cell lung carcinoma) behaviour.[9]
- They are thought to arise from pulmonary neuroendocrine cells.[10]
Classification
The grouping can be divided into four types:[11]
- Small cell carcinoma.
- Large cell neuroendocrine carcinoma.
- Typical carcinoid.
- Atypical carcinoid.
Cytologic features
Cytologic features useful for differentiation:
- Small cell carcinoma: necrosis, scant cytoplasm, mitoses.
- Typical carcinoid: often more cytoplasm, no necrosis, low mitotic rate (MIB1: scant staining).
- Atypical carcinoid: higher mitotic rate/MIB1 than typical carcinoid,[12] no necrosis.
Notes:[11]
- Large cell and small cell tumours behave in a similar fashion; large cell can be considered a morphological variant of small cell.
- 9/10 of carcinoids are typical and usually have a good prognosis, i.e. do not metastasize.
- Central location (vis-a-vis peripheral location) tends favours typical carcinoid over atypical carcinoid.
Malignant tumours
Adenocarcinoma of the lung
- AKA lung adenocarcinoma.
Bronchioloalveolar carcinoma
- Abbreviated BAC.
The term is no longer used in the new classification;[13] it is now "adenocarcinoma in situ" - see lung adenocarcinoma.
Squamous cell carcinoma of the lung
Small cell carcinoma of the lung
Malignant mesothelioma
- Should not be confused with benign multicystic mesothelioma and benign papillary mesothelioma.
Malignant potential
Atypical alveolar hyperplasia
General
- Generally considered the precursor lesion to adenocarcinoma in situ.[16]
- Typically an incidental finding, i.e. asymptomatic.[17]
Microscopic
Features:[17]
- Enlarged alveolar lining cells with:
- Hobnail morphology - free (luminal) surface area > attached/basal surface area.
- Hyperchromasia.
- Limited extent:
- <5 mm. †
DDx:
- Adenocarcinoma in situ.
- Lung adenocarcinoma.
Note:
Image:
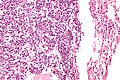
Atypical carcinoid lung tumour
- AKA atypical carcinoid tumour of the lung.
General
- Approximately 20% of lung carcinoids.[18]
Microscopic
Features:[19]
- Nests of cells.
- Stippled chromatin.
- Mild-to-moderate amount of cytoplasm.
- No necrosis/focal necrosis.
- Moderate mitotic rate (2-10/HPF - definition suffers from HPFitis).
DDx:
IHC
- MIB1 moderate staining.
Solitary fibrous tumour of the pleura
Benign tumours
Pulmonary carcinoid tumourlet
- AKA carcinoid tumourlet.
General
- Neuroendocrine cell proliferation.[20]
- Essentially a small typical carcinoid.
Microscopic
Features:
- Nests of cells - classic pattern.
- Salt and pepper chromatin - key feature.
- Size criterion: <= 4 mm.[21]
DDx:
Images:
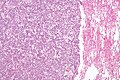
Typical carcinoid lung tumour
General
- Approximately 80% of lung carcinoids.[18]
Presentation:[22]
- Cough.
- Hemoptysis.
Gross
- Well-circumscribed, solid.
- Location - central airways (85%), remainder peripheral.[23]
Microscopic
Features:
- Nests of cells.
- Stippled chromatin.
- Moderate cytoplasm.
- No necrosis.
- Low mitotic rate.
- Size criterion: > 4 mm.[21]
DDx:
Images
IHC
- MIB1 scant staining.
Clear cell sugar tumour of the lung
- AKA clear cell sugar tumour.
- Abbreviated CCST.
General
Microscopic
Features:[25]
- Sheets or trabeculae.
- Irregular epithelioid cells with:
- Focally clear cytoplasm.
Images:
IHC
- HMB-45 +ve (nuclear & cytoplasmic).
See also
- Lung - introduction to the lung, includes a basic approach.
- Medical lung disease.
- Thyroid.
- Basics.
- Heart.
References
- ↑ Righi L, Volante M, Rapa I, Scagliotti GV, Papotti M (August 2007). "Neuro-endocrine tumours of the lung. A review of relevant pathological and molecular data". Virchows Arch. 451 Suppl 1: S51–9. doi:10.1007/s00428-007-0445-0. PMID 17684766.
- ↑ URL: http://www.nature.com/modpathol/journal/v21/n2s/full/3801018a.html. Accessed on: 8 September 2010.
- ↑ Lutschg JH (January 2009). "Lung cancer". N. Engl. J. Med. 360 (1): 87-8; author reply 88. doi:10.1056/NEJMc082208. PMID 19118313.
- ↑ 4.0 4.1 Jagirdar J (March 2008). "Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung". Arch. Pathol. Lab. Med. 132 (3): 384-96. PMID 18318581. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=132&page=384.
- ↑ Hiroshima K, Iyoda A, Shida T, et al (October 2006). "Distinction of pulmonary large cell neuroendocrine carcinoma from small cell lung carcinoma: a morphological, immunohistochemical, and molecular analysis". Mod. Pathol. 19 (10): 1358-68. doi:10.1038/modpathol.3800659. PMID 16862075.
- ↑ URL: http://jcp.bmjjournals.com/content/58/9/978.full. Accessed: 11 February 2010.
- ↑ Al-Zahrani IH (July 2008). "The value of immunohistochemical expression of TTF-1, CK7 and CK20 in the diagnosis of primary and secondary lung carcinomas". Saudi Med J 29 (7): 957-61. PMID 18626520.
- ↑ Compérat E, Zhang F, Perrotin C, et al. (October 2005). "Variable sensitivity and specificity of TTF-1 antibodies in lung metastatic adenocarcinoma of colorectal origin". Mod. Pathol. 18 (10): 1371–6. doi:10.1038/modpathol.3800422. PMID 15861215. http://www.nature.com/modpathol/journal/v18/n10/full/3800422a.html.
- ↑ URL: http://emedicine.medscape.com/article/426400-overview. Accessed on: 20 January 2010.
- ↑ Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS (2006). "Neuroendocrine tumors of the lung: clinical, pathologic, and imaging findings". Radiographics 26 (1): 41–57; discussion 57–8. doi:10.1148/rg.261055057. PMID 16418242.
- ↑ 11.0 11.1 URL: http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_is_lung_carcinoid_tumor_56.asp. Accessed on: 16 February 2011.
- ↑ Geddie, W. February 2010.
- ↑ Travis, WD.; Brambilla, E.; Noguchi, M.; Nicholson, AG.; Geisinger, K.; Yatabe, Y.; Powell, CA.; Beer, D. et al. (Sep 2011). "International association for the study of lung cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary.". Proc Am Thorac Soc 8 (5): 381-5. doi:10.1513/pats.201107-042ST. PMID 21926387.
- ↑ Travis, WD. (Oct 2010). "Advances in neuroendocrine lung tumors.". Ann Oncol 21 Suppl 7: vii65-71. doi:10.1093/annonc/mdq380. PMID 20943645.
- ↑ Mori, M.; Rao, SK.; Popper, HH.; Cagle, PT.; Fraire, AE. (Feb 2001). "Atypical adenomatous hyperplasia of the lung: a probable forerunner in the development of adenocarcinoma of the lung.". Mod Pathol 14 (2): 72-84. doi:10.1038/modpathol.3880259. PMID 11235908.
- ↑ 16.0 16.1 Sakuma, Y.; Matsukuma, S.; Yoshihara, M.; Nakamura, Y.; Nakayama, H.; Kameda, Y.; Tsuchiya, E.; Miyagi, Y. (Sep 2007). "Epidermal growth factor receptor gene mutations in atypical adenomatous hyperplasias of the lung.". Mod Pathol 20 (9): 967-73. doi:10.1038/modpathol.3800929. PMID 17618248.
- ↑ 17.0 17.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 114. ISBN 978-0781765275.
- ↑ 18.0 18.1 Naalsund, A.; Rostad, H.; Strøm, EH.; Lund, MB.; Strand, TE. (Apr 2011). "Carcinoid lung tumors--incidence, treatment and outcomes: a population-based study.". Eur J Cardiothorac Surg 39 (4): 565-9. doi:10.1016/j.ejcts.2010.08.036. PMID 20888248.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 115. ISBN 978-0781765275.
- ↑ Bennett, GL.; Chew, FS. (Mar 1994). "Pulmonary carcinoid tumorlets.". AJR Am J Roentgenol 162 (3): 568. PMID 8109497.
- ↑ 21.0 21.1 URL: http://pathhsw5m54.ucsf.edu/case7/image75.html. Accessed on: 23 January 2012.
- ↑ Gungor, S.; Damadoglu, E.; Aybatli, A.; Yilmaz, A.; Kir, A.; Akkaya, E. (Jul 2006). "Typical pulmonary carcinoid tumors: presentation and outcome of 24 cases.". Med Sci Monit 12 (7): CR315-8. PMID 16810137.
- ↑ Meisinger, QC.; Klein, JS.; Butnor, KJ.; Gentchos, G.; Leavitt, BJ. (Nov 2011). "CT features of peripheral pulmonary carcinoid tumors.". AJR Am J Roentgenol 197 (5): 1073-80. doi:10.2214/AJR.10.5954. PMID 22021498.
- ↑ Demirci, I.; Herold, S.; Kopp, A.; Flaßhove, M.; Klosterhalfen, B.; Janßen, H. (2012). "Overdiagnosis of a typical carcinoid tumor as an adenocarcinoma of the lung: a case report and review of the literature.". World J Surg Oncol 10: 19. doi:10.1186/1477-7819-10-19. PMID 22269186.
- ↑ 25.0 25.1 25.2 Kim, WJ.; Kim, SR.; Choe, YH.; Lee, KY.; Park, SJ.; Lee, HB.; Chung, MJ.; Jin, GY. et al. (Dec 2008). "Clear cell "sugar" tumor of the lung: a well-enhanced mass with an early washout pattern on dynamic contrast-enhanced computed tomography.". J Korean Med Sci 23 (6): 1121-4. doi:10.3346/jkms.2008.23.6.1121. PMC 2610653. PMID 19119463. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2610653/.