Uterine cervix
The uterine cervix, also simply cervix, is the gateway to the uterine corpus. It is not infrequently afflicted by cancer -- squamous cell carcinoma. Prior to routine Pap tests it was a leading cause of cancer death in women in the Western world.
Polyps associated with the cervix are discussed the cervical polyp article.
Cytopathology of the uterine cervix is dealt with in the gynecologic cytopathology article.
Introduction
Overview
- Most cervix cancer is squamous cell carcinoma.
- An effective screening test to detect this is the Pap test, which is dealt with in the gynecologic cytopathology article.
- The work-up of a suspicious Pap test is a colposcopic examination and biopsies, which are the topic of this article.
Indications for coloposcopic exam (based on the ASCCP Consensus Guidelines of 2001):[1]
- High-grade squamous intraepithelial lesion (HSIL).
- Repeated low-grade squamous intraepithelial lesion (LSIL).
- Atypical squamous cells of undetermined significance (ASCUS) and a positive HPV test.
- ASC-H.
- Atypical glandular cells (AGC) not otherwise specified.
- Adenocarcinoma in situ (AIS).
Colposcopic examination
- Performed by gynecologists.
- Exam usually includes a search for acetowhite epithelium (AWE); this is accomplished by the application of acetic acid (to help identify lesions for biopsy).
- Neoplastic cervical lesions are typically white.[2]
- Squamous metaplasia is also white.[3]
- Cervical ectropian (AKA cervical eversion, AKA ectropian) = endocervical epithelium at external os, considered benign, grossly has a granulation tissue-like appearance.[4]
Cervical specimens
Cytology
- Pap test - see gynecologic cytopathology.
Biopsies
The types of biopsies that are done are:
- Cervical biopsies - prompted by abnormal Pap test, e.g. HSIL, to look for squamous cell carcinoma of the uterine cervix.
- Endocervical curettage (ECC) - to work-up columnar dysplasia, e.g. endocervical adenocarcinoma/endometrial adenocarcinoma.
Surgical specimens
- Loop electrosurgical excision procedure (LEEP).
- Radical trachelectomy - removal of the uterine cervix and parametria, preserves fertility.
- Radical hysterectomy - advanced cervical carcinoma (Stage IA2 and Stage IB1), recurrent carcinoma.[5]
Other
- Total abdominal hysterectomy - for non-cervical pathology, e.g. uterine leiomyomas, uterine adenomyosis.
- Radical hysterectomy - for endometrial carcinoma with endocervical involvement.
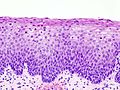
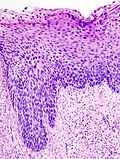
Normal histology
- The uterine cervix consists of non-keratinized squamous epithelium and simple columnar epithelium.
- The area of overlap (between squamous & columnar) is known as the "transformation zone".[6]
- Also known as "transition zone".
Images:
Negative LEEP
Transformation zone - biopsy
Microscopic
Features:
- Small round cells.
- No halos.
- No nuclear membrane irregularities.
Images:
Sign out
UTERINE CERVIX, BIOPSY: - TRANSFORMATION ZONE WITHOUT APPARENT PATHOLOGY. - NEGATIVE FOR DYSPLASIA.
UTERINE CERVIX, BIOPSY: - SQUAMOUS MUCOSA WITHOUT APPARENT PATHOLOGY. - STRIPPED ENDOCERVICAL EPITHELIUM WITHOUT APPARENT PATHOLOGY. - NEGATIVE FOR DYSPLASIA.
Endocervical glands
Microscopic
Features: Cervical glands normally have round nuclei and vaguely resemble the colonic mucosa.
Notes:
- If the nuclei are columnar think cancer! This is like in the colon-- columnar nuclei = badness.
- Memory device: The Cs (Cervix & Colon) are similar.
- Endocervical epithelium (ECE) has a morphology similar to the epithelium of secretory phase endometrium (SPE):
- ECE - grey foamy appearing cytoplasm.
- SPE - eosinophilic cytoplasm.
- Most useful feature to differentiate ECE and SPE is the accompanying stroma.
Sign out
UTERINE ENDOCERVIX, CURETTAGE: - ENDOCERVICAL MUCOSA AND STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS.
Inflamed with squamous epithelium
UTERINE ENDOCERVIX, CURETTAGE: - BENIGN STRIPPED ENDOCERVICAL EPITHELIUM AND SCANT INFLAMED ENDOCERVICAL MUCOSA. - VERY SCANT SUPERFICIAL SQUAMOUS EPITHELIUM WITHOUT APPARENT PATHOLOGY.
Squamous epithelium present
UTERINE ENDOCERVIX, CURETTAGE: - ENDOCERVICAL MUCOSA WITHIN NORMAL LIMITS. - SQUAMOUS EPITHELIUM WITHOUT APPARENT PATHOLOGY.
Endometrium present
UTERINE ENDOCERVIX, CURETTAGE: - ENDOCERVICAL MUCOSA WITHIN NORMAL LIMITS. - SCANT NON-PROLIFERATIVE ENDOMETRIUM.
Inflamed
UTERINE ENDOCERVIX, CURETTAGE: - INFLAMED ENDOCERVICAL MUCOSA. - REACTIVE SQUAMOUS EPITHELIUM. - NEGATIVE FOR MALIGNANCY.
UTERINE ENDOCERVIX, CURETTAGE: - BENIGN INFLAMED ENDOCERVICAL MUCOSA. - STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS.
No stroma present
UTERINE ENDOCERVIX, CURETTAGE: - STRIPPED ENDOCERVICAL EPITHELIUM WITHOUT APPARENT PATHOLOGY.
Limited tissue
UTERINE ENDOCERVIX, CURETTAGE: - ONE MINUTE FRAGMENT OF ENDOCERVICAL EPITHELIUM WITHOUT APPARENT PATHOLOGY, SEE COMMENT. - VERY SCANT SUPERFICIAL SQUAMOUS EPITHELIUM WITHOUT APPARENT PATHOLOGY. COMMENT: The assessment is severely limited by the small amount of tissue. A re-biopsy should be considered within the clinical context.
UTERINE ENDOCERVIX, CURETTAGE: - ONE MINUTE FRAGMENT OF ENDOCERVICAL EPITHELIUM WITHOUT APPARENT PATHOLOGY, SEE COMMENT. - VERY SCANT SUPERFICIAL SQUAMOUS EPITHELIUM WITHOUT APPARENT PATHOLOGY. COMMENT: The assessment is severely limited by the small amount of tissue. Clinical correlation is suggested.
UTERINE ENDOCERVIX, CURETTAGE: - BENIGN SQUAMOUS EPITHELIUM WITH METAPLASTIC CHANGE. - VERY SCANT BENIGN ENDOCERVICAL EPITHELIUM, SUBOPTIMAL SAMPLING.
UTERINE CERVIX, BIOPSY: - MINUTE FRAGMENTS OF SUPERFICIAL SQUAMOUS EPITHELIUM WITHOUT APPARENT PATHOLOGY. - SCANT MUCOUS AND INFLAMMATORY CELLS. - SEE COMMENT. COMMENT: The assessment is severely limited by the small amount of tissue. A re-biopsy should be considered within the clinical context.
Inadequate biopsy
- Unfortunately, inadequate biopsies are common.
Endocervix
Sign out
No endocervical epithelium
UTERINE ENDOCERVIX, CURETTAGE: - SQUAMOUS EPITHELIUM WITHOUT APPARENT PATHOLOGY. - NO ENDOCERVICAL EPITHELIUM IDENTIFIED. - MUCOUS AND INFLAMMATORY CELLS.
No epithelium
UTERINE ENDOCERVIX, CURETTAGE: - MUCOUS AND INFLAMMATORY CELLS. - NO EPITHELIUM IDENTIFIED.
No tissue
UTERINE ENDOCERVIX, CURETTAGE: - NO TISSUE PRESENT, SEE COMMENT. COMMENT: No tissue identified on gross or microscopy.
UTERINE ENDOCERVIX, CURETTAGE: - NO TISSUE PRESENT, SEE COMMENT. COMMENT: No tissue identified on microscopy. No tissue is seen on inspection of the paraffin block.
Where to start
- Identify epithelium - exocervical (stratified squamous), endocervical (simple columnar), both.
- If there is both exocervix and endocervix --> transition zone.
- Identify possible squamous lesions.
- Identify possible endocervical lesions.
Benign (common)
Nabothian cyst
General
- Benign.
- Common.
Gross
- Bump.
- Pale colour.
DDx - clinical:
Image:
Microscopic
Features:
- Simple endocervical cyst.
- Usually lined by endocervical epithelial cells - may be flattened.
- Columnar morphology with large clear, apical vacuoles.
- +/-Macrophages.
- +/-Mucus.
- Usually lined by endocervical epithelial cells - may be flattened.
Note:
- May be lined by tubal epithelium.
- Cilia.
- High NC ratio ~ 1:1.[citation needed]
Image:
Sign out
CERVICAL POLYP, REMOVAL: - BENIGN POLYPOID FRAGMENT OF EXOCERVICAL MUCOSA WITH NABOTHIAN CYSTS AND BENIGN ENDOCERVICAL EPITHELIUM.
POLYPOID LESION ("CERVICAL POLYP"), EXCISION:
- POLYPOID NABOTHIAN CYST.
Tunnel cluster
General
- Benign.[7]
- Not the same as microglandular hyperplasia.[8]
- Considered a special type of nabothian cyst.[9]
Microscopic
- Well-circumscribed lesion consisting of:
- Benign endocervical glands.
- Dilated & filled with mucin or (less commonly) eosinophilic secretions.
- Lining epithelium compressed/flattened (attenuated).
- Gland architecture: branching, tortuous.
- Scant intervening stroma.
Notes:
- Usually no nuclear atypia and no mitotic activity.
- Important only as one could possibly mistake it as minimal deviation adenocarcinoma, AKA adenoma malignum.[12]
Images:
Microglandular hyperplasia
- Not to be confused with microglandular adenosis.
- Abbreviated MGH.
- AKA microglandular change.
General
Microscopic
Features:[8]
- Cytologically benign - important.
- Usually cuboidal morphology.
- Typically clear cytoplasm.
- Crowded small glands (classic), reticular or solid.
Significant negatives:
- Nuclear atypia absent.
- NC ratio not significantly increased.
DDx:
- Adenocarcinoma in situ of the uterine cervix.
- Endometrial mucinous microglandular adenocarcinoma - very rare.[13][14]
- Clear cell carcinoma of the uterine cervix.[15]
Images:
IHC
Features:[17]
- Ki-67 ~ 0.5% cells.
- Vimentin -ve.
- PR +ve ~ 60% of cases.
- ER +ve/-ve.
Others:[17]
- p53 -ve.
- CEA -ve.
- p16 -ve (scattered cells +ve).[citation needed]
Sign out
UTERINE ENDOCERVIX, CURETTAGE: - MICROGLANDULAR HYPERPLASIA. - SQUAMOUS EPITHELIUM WITH REACTIVE CHANGES AND METAPLASTIC CHANGES.
Micro
The sections show gland forming epithelium without nuclear atypia (no nuclear membrane irregularities, no coarse chromatin). The nuclei are less than 2x the size of a neutrophil, regularly spaced, pale staining and have small regular nucleoli visible with the 20x objective. No mitotic activity is apparent.
Fragments of reactive squamous epithelium with metaplastic changes are present. Benign superficial squamous epithelium is identified.
Wolffian duct hyperplasia
General
- Benign.
Microscopic
Features:
- Abundant small tubules with a simple cuboidal epithelium.
- Round small bland nucleus.
DDx:
Stains
- PAS-D+ve (cytoplasm).
Squamous metaplasia of the uterine cervix
- Abbreviated SMC.
General
- Benign process: columnar cells -> squamoid cells.
- Biologic response to irritation and/or inflammation.
Gross
Microscopic
Features:
- Uniform cell spacing - no crowding - key feature.
- Nuclei are uniform size and round.
- Nucleoli present.
- Distinct cell borders
- +/-Intercellular bridges (due to edema) - common.
- Adjacent/closely associated with columnar epithelium.
- Columnar epithelium superficial in immature metaplasia.
Negatives:
- No mitoses (think cancer/CIN if you see 'em).
- Usually no hyperchromatism (think cancer/CIN if you see it).
Notes:
- NC ratio high - possible to confuse with CIN III.
- May have goblet cells - uncommon.[18]
DDx:
- CIN II - esp. for immature squamous metaplasia.
- CIN III.
- Squamous cell carcinoma of the uterine cervix.
Images:
- Squamous metaplasia - cervix (sciencephoto.com).
- Squamous metaplasia - bronchus (WC).
- Squamous metaplasia - cytology (techriver.net).
- Immature squamous metaplasia (eurocytology.eu).[19]
IHC
- p16 weak-to-moderate patchy +ve -- checkerboard-like; not full thickness.
- Strong diffuse full thickness positivity in HSIL and SCC.
- Ki-67 - low proliferative rate.
Sign out
ECC
UTERINE ENDOCERVIX, CURETTAGE: - SQUAMOUS METAPLASTIC EPITHELIUM. - VERY SCANT STRIPPED ENDOCERVICAL EPITHELIUM.
Cervical biopsy
UTERINE CERVIX, BIOPSY: - SQUAMOUS METAPLASTIC EPITHELIUM. - SCANT BENIGN ENDOCERVICAL GLANDS.
UTERINE CERVIX, BIOPSY: - SQUAMOUS METAPLASTIC EPITHELIUM. - SCANT BENIGN ENDOCERVICAL GLANDS. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY.
Micro
The sections show stratified squamous epithelium. The cells are equally spaced and spaces are seen between the cells (edema).
The nuclei are not significantly enlarged (<3x resting lymphocyte diameter). No nuclear halos are apparent. The nuclear membranes are regular. Mild inflammation is present. Nucleoli are present focally.
No endocervical cells are identified.
Reactive squamous epithelium of the uterine cervix
General
- Common.
Microscopic
Features:
- Inflammation - key feature.
- Lymphocytes.
- Plasma cells.
- Mild nuclear enlargement. †
- Nucleoli - important.
Note:
- † Normal squamous cell nuclei are approximately 8 μm.[20]
- Mild enlargement ~ 2-3x normal.
- CIN I nuclei are ~ 3x normal (24 μm).
DDx:
IHC
- p16 -ve.
Sign out
UTERINE CERVIX, BIOPSY: - REACTIVE SQUAMOUS EPITHELIUM. - BENIGN ENDOCERVICAL GLANDS. - NEGATIVE FOR MALIGNANCY.
COMMENT: The squamous epithelium is negative for p16 staining. Ki-67 staining is predominantly in the lower third of the epithelium.
Tubal metaplasia of the uterine cervix
- AKA tubal metaplasia, abbreviated TM.
General
- Benign.
- Mimics the appearance of AIS - especially at low power.
Microscopic
Features - like the fallopian tube:
- Nuclear crowding vis-à-vis benign endocervical epithelium (low power).
- Mixed cell population (high power):
- Peg cells - "tall" and "skinny".
- Columnar/golf tee-like appearance.
- Ciliated cells - cilia, pale cytoplasm, round central nucleus.
- Secretory cells - non-ciliated, basophilic cytoplasm, round small basal nuclei.
- Peg cells - "tall" and "skinny".
DDx:
Image:
IHC
Features:[21]
- Vimentin +ve.
- CEA -ve/+ve.
- p16 -ve.[citation needed]
Non-invasive
Cervical intraepithelial neoplasia
- CIN I, CIN II and CIN III redirect to here.
- Abbreviated CIN.
General
- Refers to changes in squamous epithelium.
Grades (squamous intraepithelial neoplasia):
- CIN I = mild dysplasia.
- CIN II = moderate dysplasia.
- CIN III = severe dysplasia.
Bethesda system:
- LSIL (low-grade squamous intraepithelial lesion) = CIN I.
- HSIL (high-grade squamous intraepithelial lesion) = CIN II, CIN III.
Treatment
Loop electrosurgical excision procedure (LEEP):
- Used for squamous lesions -- pathologist typically gets several pieces.
Cone:
- Used for endocervical lesions, i.e. adenocarcinoma in situ (AIS).
- Pathologist gets a ring or donut-shaped piece of tissue.
Gross
- Acetowhite lesion at colposcopy.
Microscopic
Cervical intraepithelial neoplasia I
Features - Cervical intraepithelial neoplasia (CIN) I:[22]
- "Koilocytic atypia":[23]
Note:
- Atypical cells usually close to basement membrane.
- May be seen, focally, in the upper layers.[23]
Image
www:
Cervical intraepithelial neoplasia II
Features - CIN II:[22]
- Increased nuclear-cytoplasmic ratio, loss of polarity, incr. mitoses, hyperchromasia.
- If there are large nuclei... you should seen 'em on low power, i.e. 25x.
Image:
Cervical intraepithelial neoplasia III
Features - CIN III:[22]
- Same changes as in CIN II + outer third (or full thickness).
Notes:
- Hyperchromasia is a very useful feature for identifying CIN (particularly at low power, i.e. 25x).
- Koilocytes are the key feature of CIN I.
- Koilocytes are not considered to be part of a CIN II lesion or CIN III lesion.
- Large irregular nuclei are not required for CIN II... but you should think about it.
- Some mild changes at the squamo-columnar junction are expected.
- Look for the location of mitoses...
- If there is a mitosis in the inner third (of the epithelial layer) = think CIN I.
- If there is a mitosis in the middle third (of the epithelial layer) = think CIN II.
- If there is a mitosis in the outer third = think CIN III.
- Prominent nucleoli are not present in CIN.[23]
- Nucleoli are common in reactive changes.[25]
- The most probably place for CIN is the posterior cervix (6 o'clock position) - risk is marginally increased.[26]
DDx:
Images
www:
- CIN III (flickr.com/euthman).
- CIN III (flickr.com/euthman).
- CIN III - several images (flickriver.com).
Koilocytes versus benign squamous
Koilocytes:
- Perinuclear clearing.
- Nuclear changes.
- Size similar (or larger) to those in the basal layer of the epithelium.
- Nuclear enlargement should be evident on low power, i.e. 25x. [27]
- Central location - nucleus should be smack in the middle of the cell.
Notes:
- Both perinuclear clearing and nuclear changes are essential.
- Benign cells have a small nucleus that is peripheral.
IHC
Features:[28]
- p16.
- Diffuse strong staining involving at least all of the basal aspect of the epithelium = CIN II or CIN III.
- Patchy, weak positive staining = CIN I or squamous metaplasia.
- Ki-67.
- Several positive cells above basal layer suggests CIN II or CIN III.
Notes:
- Both p16 and Ki-67 are usually negative in CIN I -- 75% of cases.[29]
- CIN I with p16 staining appears to have a higher risk of progression the p16 negative CIN I.[30]
Sign-out
ECC - cannot grade
UTERINE CERVIX, BIOPSY: - FRAGEMENTS OF SQUAMOUS EPITHELIUM SHOWING DYSPLASIA, SEE COMMENT. COMMENT: The fragments of squamous epithelium do not show full epithelial thickness. Thus, while dysplasia is apparent, it is not possible to distinguish low-grade from high-grade in this specimen. That said, there is at least low grade-dysplasia. Follow-up is recommended with re-biopsy if clinically indicated.
LEEP
UTERINE CERVIX, LOOP ELECTROSURGICAL EXCISION PROCEDURE (LEEP): - CERVICAL INTRAEPITHELIAL NEOPLASIA 2 (MODERATE DYSPLASIA). - DEEP, ENDOCERVICAL AND EXOCERVICAL MARGINS NEGATIVE FOR INTRAEPITHELIAL NEOPLASIA.
UTERINE CERVIX, LOOP ELECTROSURGICAL EXCISION PROCEDURE (LEEP): - CERVICAL INTRAEPITHELIAL NEOPLASIA 3 (SEVERE DYSPLASIA). - DEEP, ENDOCERVICAL AND EXOCERVICAL MARGINS NEGATIVE FOR INTRAEPITHELIAL NEOPLASIA.
UTERINE CERVIX, LOOP ELECTROSURGICAL EXCISION PROCEDURE (LEEP): - CERVICAL INTRAEPITHELIAL NEOPLASIA 3 (SEVERE DYSPLASIA). - DEEP, ENDOCERVICAL AND EXOCERVICAL MARGINS NEGATIVE FOR INTRAEPITHELIAL NEOPLASIA. - NEGATIVE FOR MALIGNANCY. COMMENT: CIN 3 is seen in 2 of 5 blocks and has a total linear extent of 17 millimeters.
UTERINE CERVIX, LOOP ELECTROSURGICAL EXCISION PROCEDURE (LEEP): - HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (HSIL). - DEEP, ENDOCERVICAL AND EXOCERVICAL MARGINS NEGATIVE FOR DYSPLASIA. - NEGATIVE FOR MALIGNANCY. COMMENT: HSIL is seen in 3 of 4 blocks and has a total linear extent of approximately 12 millimeters. The HSIL is in keeping with cervical intraepithelial neoplasia 3 (severe dysplasia).
Biopsy
LSIL
UTERINE CERVIX, BIOPSY: - LOW-GRADE SQUAMOUS INTRAEPITHELIAL LESION (LSIL). - NO ENDOCERVICAL EPITHELIUM IDENTIFIED.
UTERINE CERVIX, BIOPSY: - LOW-GRADE SQUAMOUS INTRAEPITHELIAL LESION (LSIL). - TRANSFORMATION ZONE PRESENT.
UTERINE CERVIX, BIOPSY: - LOW-GRADE SQUAMOUS INTRAEPITHELIAL LESION (LSIL). - CERVICITIS, CHRONIC. - NO ENDOCERVICAL EPITHELIUM IDENTIFIED.
UTERINE CERVIX, BIOPSY: - LOW-GRADE SQUAMOUS INTRAEPITHELIAL LESION (LSIL). - TRANSFORMATION ZONE PRESENT. COMMENT: A p16 stain is patchy and confined mostly to the lower aspect of the squamous epithelium.
CIN 1
UTERINE CERVIX, BIOPSY: - CERVICAL INTRAEPITHELIAL NEOPLASIA 1 (MILD DYSPLASIA). - TRANSFORMATION ZONE PRESENT.
COMMENT: The Ki-67 positive cells are confined to the lower aspect of the squamous epithelium. A p16 stain is negative.
At least CIN 2
UTERINE CERVIX, BIOPSY: - AT LEAST CERVICAL INTRAEPITHELIAL NEOPLASIA 2 (MODERATE DYSPLASIA). - TRANSFORMATION ZONE PRESENT.
UTERINE ENDOCERVIX, CURETTAGE: - HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (HSIL). - ENDOCERVICAL MUCOSA AND STRIPPED ENDOCERVICAL EPITHELIUM WITHIN NORMAL LIMITS. COMMENT: The HSIL is in keeping with CIN 2.
CIN 3
UTERINE CERVIX, BIOPSY: - CERVICAL INTRAEPITHELIAL NEOPLASIA 3 (SEVERE DYSPLASIA).
COMMENT: A p16 stain marks the full thickness of the squamous epithelium and is strong. A Ki-67 stain marks increased numbers of superficial epithelial cells.
Micro
CIN 1
The sections show the transformation zone. The squamous epithelium has cells with an increased nuclear size, nuclear hyperchromasia, perinuclear clearing and irregularities in the nuclear membrane. The nucleus-to-cytoplasm ratio is mildly increased. Occasional binucleation is identified. Mitoses are seen in the low third of the epithelium. Nucleoli are not apparent. No columnar dysplasia is identified.
CIN 3
The sections show the transformation zone.
The squamous epithelium has an increased nuclear-cytoplasmic ratio, loss of polarity, mitoses and nuclear hyperchromasia extending to the superficial third of the epithelium. Mitoses are seen in the upper third of the epithelium. No nucleoli are present. No invasion is identified.
The columnar epithelium has focal involvement by the squamous lesion. There is no columnar dysplasia. The margins are negative for dysplasia.
Biopsy
The sections show the transformation zone.
The squamous epithelium has an increased nuclear-cytoplasmic ratio, loss of polarity, mitoses and nuclear hyperchromasia extending to the superficial third of the epithelium. Mitoses are seen in the upper third of the epithelium. Nucleoli are not apparent. No invasion is identified.
No columnar dysplasia is identified.
Alternate
The sections show fragments of transformation zone.
There is dysplastic squamous epithelium with coarse chromatin, nuclear hyperchromasia, nuclear enlargement, irregular nuclear membranes, and an increase nuclear-to-cytoplasmic ratio. Mitotic activity is abundant focally (5 mitoses/0.2376 mm*mm). The dysplastic squamous epithelium does not show appreciable maturation toward the surface (CIN 3). The dysplastic squamous epithelium is not associated with stroma; thus, the presence/absence of invasion cannot be assessed. Small nucleoli are seen rarely.
There is benign squamous epithelium. Scant benign stripped endocervical epithelium is present.
Endocervical adenocarcinoma in situ
- For the cytology see Gynecologic cytopathology#Endocervical adenocarcinoma in situ
- AKA adenocarcinoma in situ, abbreviated AIS.
General
- Usually due to HPV.
- May be found together with squamous neoplasias of the cervix.
- AIS of the cervix is much less common than squamous dysplasia of the cervix/SCC of the cervix.
- Generally, definitely diagnosed with an endocervical curettage (ECC).
Gross
- Not apparent at colposcopy.
Microscopic
Features:[8]
- Nuclear changes - key feature:
- Variable nuclear stratification.
- Nuclear crowding/pseudostratification.
- Nuclear enlargement.
- Often cigar-shaped nuclei.
- Coarse chromatin.
- Small nucleolus or nucleoli.
- Variable nuclear stratification.
- +/-Mitoses.
- +/-Reduced cytoplasmic mucin.
- Preservation of glandular architecture.
- Normal gland spacing - lack of complexity ("lobular pattern").
- Normal gland depth (subjective).
DDx:
- Tubal metaplasia.
- Arias-Stella reaction.
- Endometriosis.
- Lower uterine segment epithelium[31] - esp. proliferative phase endometrium - mitoses rare, NC ratio normal, stroma different.
- Endocervical adenocarcinoma - often has paradoxical maturation... paler cytoplasm & nuclei than adjacent AIS.
- Metastatic adenocarcinoma.
- Proliferative phase endometrium - endometrial type stroma, cytoplasm not pale staining, no nuclear atypia (smooth nuclear contour, stratified).
Images:
- Endocervical AIS adjacent to normal (flickriver.com/euthman).
- Endocervical adenocarcinoma in situ (techriver.net).
- Endocervical adenocarcinoma in situ (womenshealthsection.com).[32]
- Endocervical adenocarcinoma in situ - cytology (techriver.net).
IHC
- p16 +ve.
- CEA +ve.
- Vimentin -ve.
Cancer
Squamous cell carcinoma of the uterine cervix
- AKA cervical squamous cell carcinoma.
General
- Most common type of cervical cancer.
Risk factors:
- Low socioeconomic status.
- Smoking.
- Early first intercourse.
- High risk partners.
- Human papillomavirus (HPV) infection, esp. "high risk HPV".
- HPV 16 closely assoc. with SCC.[33]
Microscopic
Features:
- Squamous differentiation.
- +/-Intracellular bridges.
- Scant-to-moderate cytoplasm.
- Penetration of basement membrane.
- May be challenging to determine.
- Nuclear atypia.
SCC of the cervix versus CIN III: Invasive cancer look for:
- Eosinophilia.
- Extra large nuclei, i.e. nuclei 5x normal size.
- Stromal inflammation (lymphocytes, plasma cells).
- Long rete ridges.
- Numerous beeds/blobs of epithelial cells that seem unlikely to be rete ridges.
- Desmoplastic stroma - increased cellularity, spindle cell morphology.
DDx:
- Squamous metaplasia of the uterine cervix - if you can trace the squamous cells from a gland to the surface it is less likely to be invasive cancer.[34]
- CIN III +/- endocervical gland involvement.
Images:
- Microinvasive cervical SCC - low mag. (sunnybrook.ca).[35]
- Microinvasive cervical SCC - high mag. (sunnybrook.ca).[35]
- Cervical SCC - low mag. (ucsf.edu).[36]
- Cervical SCC - high mag. (uscf.edu).
Grading
Divided into:[37]
- Well-differentiated (keratinizing).
- Moderately differentiated (nonkeratinizing).
- Poorly differentiated.
Depth measurement
- Basement membrane (where it invades) to deepest point.
Note:
- Stage Ib - clinical diagnosis.
- Definition of stage Ib: clinically visible.
FIGO
Microinvasive SCC as per FIGO:
- Depth < 5 mm.
- Width < 7 mm.
- +/-Vascular invasion.
SGO
Microinvasive SCC as per The Society of Gynecologic Oncologists (SGO):
- <= 3 mm.
- Negative for vascular invasion.
Note:
- The SGO criteria the prefered by North American gynecologists.
IHC
- Factor VIII - to look for LVI.
Sign out
Early invasive SCC - things to report:
- Depth of invasion.
- Length of tumour.
- Number of blocks with tumour.
- LVI.
- Margins.
UTERINE CERVIX, BIOPSY: - FRAGMENTS OF INVASIVE SQUAMOUS CELL CARCINOMA. -- DEPTH OF INVASION AND LENTH OF TUMOUR CANNOT BE ASSESSED. -- LYMPHOVASCULAR INVASION NOT APPARENT.
Adenocarcinoma of the uterine cervix
General
- Adenocarcinoma of the cervix is much less common than squamous dysplasia of the cervix/SCC of the cervix.
- Arises from the endocervical glands.
Microscopic
Features:
- Stromal changes - "desmoplastic stroma/desmoplastic reaction".
- Fibrosis/streaming cells.
- Gland fusion.
- Glands too deep -- very fuzzy criterion.
Notes:
- AIS changes - similar to colonic dysplasia.
- AIS may occur together with CIN.
- Not infrequently they (AIS, CIN) occur together - both are due, indirectly, to HPV infection.
- May be difficult to be certain of invasion.
- A feature suggestive of invasion is cytoplasmic eosinophilia.
DDx:
- Microglandular hyperplasia - NC ratio normal, no nuclear atypia.
- Endocervical adenocarcinoma in situ.
- Metastatic adenocarcinoma.
Images:
- Cervical adenocarcinoma - low mag. (WC).
- Cervical adenocarcinoma - intermed. mag. (WC).
- Cervical adenocarcinoma - high mag. (WC).
IHC
Features for diagnosis:
- p16 +ve.
- Ki-67 -- high.
Uterus vs. cervix:[38]
- Cervix (typically): CEA +ve, p16 +ve.
- ER -ve, PR -ve, vimentin -ve.
- Uterus (typically): vimentin +ve, ER +ve, PR +ve.
- CEA -ve, p16 -ve.
Uncommon non-invasive
Stratified mucin-producing intraepithelial lesions of the cervix
- Abbreviated SMILE (Stratified Mucin-producing Intraepithelial LEsion).
General
- Rare.
- Often accompanied by cervical intraepithelial neoplasia and adenocarcinoma in situ.[39]
Microscopic
Features:[39]
- Stratified epithelium with:
- Nuclear atypia.
- Cytoplasmic clearing or vacuoles in lesions - through-out.
DDx:
Images:
IHC
Features:
- Ki-67 high.
- Keratin 14 -ve.
- p63 +ve/-ve -- only basal if positive.
Uncommon types of cervical cancer
There are a number of uncommon type of cervical cancer.
Serous carcinoma of the uterine cervix
General
- Poor prognosis.[41]
- Extremely rare.
Microscopic
Features:
- Like other serous carcinomas.
Adenosquamous carcinoma
General
- Uncomon.
Note:
- Glassy cell carcinoma is considered to be a subtype of adenosquamous carcinoma.[42]
Microscopic
Features:
- Morphologic features of both squamous carcinoma and adenocarcinoma:
- Adenocarcinoma: gland forming or mucin vacuoles.
- Squamous carcinoma: abundant eosinophilic cytoplasm, central nucleus.
Images
Clear cell carcinoma of the uterine cervix
- AKA cervical clear cell carcinoma.
General
- Associated with diethylstilbestrol exposure in utero.[43]
- Less common in the cervix - when compared to other gynecologic sites.[44]
- More common in the endometrium.[45]
Note:
- HPV does not appear to be important in the oncogenesis;[46] however, this is not completely settled.[15]
Microscopic
Features:[15]
- Like clear cell carcinoma elsewhere:
- Clear cytoplasm - key feature.
- May be absent!
- Cells have large free/luminal surface area (hobnailing pattern) and small non-free surface.
- Moderate-to-severe nuclear pleomorphism.
- Tubular and/or cystic morphology.
- May be (simple) papillary and/or solid.
- Clear cytoplasm - key feature.
- Adenosis - typically adjacent.
DDx:
- Clear cell carcinoma of the endometrium - diagnosis based on clinico-radiologic correlation (IHC & histology not useful).[15]
- Serous carcinoma of the endometrium.
- Serous carcinoma of the uterine cervix.
IHC
- Essentially considered to be like endometrial clear cell carcinoma.[15]
Small cell carcinoma of the cervix
- Like small cell carcinoma elsewhere.
DDx:
IHC
- HPV +ve.
Adenoid basal carcinoma
- See also: Basal cell carcinoma.
General
- Good prognosis.[47]
Microscopic
Features:[47]
- Nests of cells with basaloid rim and squamoid center.
- Basaloid cells look benign.
DDx:
- Ectopic prostate gland.
Image:
Glassy cell carcinoma
General
- Rare.
- Rapid growth, poor prognosis.[48]
- Considered a subtype of adenosquamous carcinoma.[49]
Microscopic
Features:[50]
- Epithelioid cells in sheets or cords.
- Round/oval nucleus.
- One or more prominent nucleoli.
- Abundant finely vacuolated eosinophilic to amphophilic cytoplasm.
- Distinct cell borders.
- Inflammation - esp. eosinophils.[51]
DDx:
Images:
- WC:
- www:
Stains
Villoglandular adenocarcinoma of the cervix
- AKA well-differentiated papillary villoglandular adenocarcinoma,[53] AKA villoglandular papillary adenocarcinoma, AKA well-differentiated villoglandular adenocarcinoma.
General
- Rare.
- Younger patients and relatively good prognosis.[54]
- Associated with HPV.
- May also arise from the endometrium.[55]
Microscopic
Features:[56]
- Papillary structures (nipple-like shapes with a fibrovascular core) that are long.
- Nobody defines "long".
- Perhaps - long >3:1 length:width.
- Nobody defines "long".
- Covered by columnar (or cuboidal) epithelium.
- Intracellular mucin (focal).
DDx:
- Serous carcinoma of the cervix.
Images:
- www:
- WC:
Mucoepidermoid carcinoma of the uterine cervix
General
- Controversial - not in the WHO.[57]
Microscopic
Features:[58]
- Squamous cell carcinoma-like with:
- No glands formation.
- Intracellular mucin.
- Classically have mucous cells - cells with abundant fluffy cytoplasm and large mucin vacuoles - key feature.
Notes:
- Similar to the salivary gland tumour.[57]
DDx:
- Cervical intraepithelial neoplasia, i.e. CIN II, CIN III.
- Adenosquamous carcinoma.
Stains
Mucin stains:[58]
IHC
- CEA +ve.[58]
Molecular
Like the salivary gland tumour:
- t(11;19) CRTC1/MAML2.[57]
Mesonephric adenocarcinoma
General
- Arises from the mesonephric duct remnants.
Microscopic
Features:[44]
- Nuclear atypia - key feature.
- Nuclear crowding.
- Variable architecture:
- Tubular, papillary, solid, retiform (net-like[59]).
DDx:
- Mesonephric duct remnants.
- Cervical adenocarcinoma.
- Colorectal adenocarcinoma.
- Endometrioid adenocarcinoma.
IHC
Features:[44]
- CK7 +ve.
- CD10 +ve.
Others:[44]
- CK20 -ve.
- ER -ve.
- PR -ve.
- CEA -ve.
Minimal deviation adenocarcinoma of the uterine cervix
General
- Rare and difficult diagnosis.[60]
- Requires a deep sampling;[61] thus, usually diagnosed on cone biopsy or hysterectomy.
- Associated with Peutz-Jeghers syndrome.[62][63]
- Poor prognosis.[12]
Microscopic
Features:[64]
- Deep infiltrating glands - key feature.
- Desmoplastic stroma - may be subtle.
- Perivascular and/or perineural location.
- Minimal nuclear atypia.
- Abnormal gland morphology[12] / loss of lobular (gland) architecture. †
Note:
- † Not a criterion required by all pathologists.[60]
DDx:[60]
- Adenocarcinoma of the uterine cervix - has "obvious" nuclear atypia.
- Tunnel cluster.
IHC
Features:
- CEA +ve.[12]
- p16 -ve.[citation needed]
See also
References
- ↑ Dresang, LT.. "Colposcopy: an evidence-based update.". J Am Board Fam Pract 18 (5): 383-92. PMID 16148248.
- ↑ Zonios, G. (Aug 2012). "Reflectance model for acetowhite epithelium.". J Biomed Opt 17 (8): 87003-1. doi:10.1117/1.JBO.17.8.087003. PMID 23224202.
- ↑ 3.0 3.1 Li, W.; Venkataraman, S.; Gustafsson, U.; Oyama, JC.; Ferris, DG.; Lieberman, RW.. "Using acetowhite opacity index for detecting cervical intraepithelial neoplasia.". J Biomed Opt 14 (1): 014020. doi:10.1117/1.3079810. PMID 19256708.
- ↑ Casey, PM.; Long, ME.; Marnach, ML. (Feb 2011). "Abnormal cervical appearance: what to do, when to worry?". Mayo Clin Proc 86 (2): 147-50; quiz 151. doi:10.4065/mcp.2010.0512. PMC 3031439. PMID 21270291. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3031439/.
- ↑ Ware, RA.; van Nagell, JR. (2010). "Radical hysterectomy with pelvic lymphadenectomy: indications, technique, and complications.". Obstet Gynecol Int 2010. doi:10.1155/2010/587610. PMID 20871657.
- ↑ URL: http://www.med-ed.virginia.edu/Courses/path/gyn/cervix1.cfm. Accessed on: 12 May 2010.
- ↑ Nucci, MR. (Oct 2002). "Symposium part III: tumor-like glandular lesions of the uterine cervix.". Int J Gynecol Pathol 21 (4): 347-59. PMID 12352183.
- ↑ 8.0 8.1 8.2 8.3 Zaino, RJ. (Mar 2000). "Glandular lesions of the uterine cervix.". Mod Pathol 13 (3): 261-74. doi:10.1038/modpathol.3880047. PMID 10757337. Cite error: Invalid
<ref>tag; name "pmid10757337" defined multiple times with different content - ↑ Okamoto, Y.; Tanaka, YO.; Nishida, M.; Tsunoda, H.; Yoshikawa, H.; Itai, Y.. "MR imaging of the uterine cervix: imaging-pathologic correlation.". Radiographics 23 (2): 425-45; quiz 534-5. PMID 12640157.
- ↑ URL: http://pathologyoutlines.com/cervix.html#tunnelclusters. Accessed on: 27 February 2011.
- ↑ URL: http://surgpath4u.com/caseviewer.php?case_no=477. Accessed on: 5 September 2011.
- ↑ 12.0 12.1 12.2 12.3 Gilks CB, Young RH, Aguirre P, DeLellis RA, Scully RE (September 1989). "Adenoma malignum (minimal deviation adenocarcinoma) of the uterine cervix. A clinicopathological and immunohistochemical analysis of 26 cases". Am. J. Surg. Pathol. 13 (9): 717–29. PMID 2764221. Cite error: Invalid
<ref>tag; name "pmid2764221" defined multiple times with different content - ↑ Giordano, G.; D'Adda, T.; Gnetti, L.; Merisio, C.; Melpignano, M. (Jan 2006). "Endometrial mucinous microglandular adenocarcinoma: morphologic, immunohistochemical features, and emphasis in the human papillomavirus status.". Int J Gynecol Pathol 25 (1): 77-82. PMID 16306789.
- ↑ Zamecnik, M.; Skalova, A.; Opatrny, V. (Jun 2003). "Microglandular adenocarcinoma of the uterus mimicking microglandular cervical hyperplasia.". Ann Diagn Pathol 7 (3): 180-6. PMID 12808571.
- ↑ 15.0 15.1 15.2 15.3 15.4 Offman, SL.; Longacre, TA. (Sep 2012). "Clear cell carcinoma of the female genital tract (not everything is as clear as it seems).". Adv Anat Pathol 19 (5): 296-312. doi:10.1097/PAP.0b013e31826663b1. PMID 22885379.
- ↑ URL: http://sunnybrook.ca/content/?page=Dept_LabS_APath_GynPath_ImgAt_Cvx_neo_micro. Accessed on: 25 February 2012.
- ↑ 17.0 17.1 Qiu, W.; Mittal, K. (Jul 2003). "Comparison of morphologic and immunohistochemical features of cervical microglandular hyperplasia with low-grade mucinous adenocarcinoma of the endometrium.". Int J Gynecol Pathol 22 (3): 261-5. doi:10.1097/01.PGP.0000071043.12278.8D. PMID 12819393.
- ↑ Sivridis, E.; Karpathiou, G.; Malamou-Mitsi, V.; Giatromanolaki, A. (2010). "Intestinal-type metaplasia in the original squamous epithelium of the cervix.". Eur J Gynaecol Oncol 31 (3): 319-22. PMID 21077478.
- ↑ URL: http://www.eurocytology.eu/static/eurocytology/eng/cervical/LP1ContentAcontD.html. Accessed on: 9 October 2013.
- ↑ URL: http://www.curran.pwp.blueyonder.co.uk/cytology.htm. Accessed on: 5 November 2012.
- ↑ Marques, T.; Andrade, LA.; Vassallo, J. (Jun 1996). "Endocervical tubal metaplasia and adenocarcinoma in situ: role of immunohistochemistry for carcinoembryonic antigen and vimentin in differential diagnosis.". Histopathology 28 (6): 549-50. PMID 8803599.
- ↑ 22.0 22.1 22.2 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1075-6. ISBN 0-7216-0187-1.
- ↑ 23.0 23.1 23.2 Nucci, Marisa R.; Oliva, Esther (2009). Gynecologic Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 146. ISBN 978-0443069208.
- ↑ Roteli-Martins CM, Derchain SF, Martinez EZ, Siqueira SA, Alves VA, Syrjänen KJ (2001). "Morphological diagnosis of HPV lesions and cervical intraepithelial neoplasia (CIN) is highly reproducible". Clin Exp Obstet Gynecol 28 (2): 78–80. PMID 11491378.
- ↑ STC. January 2009.
- ↑ Pretorius, RG.; Zhang, X.; Belinson, JL.; Zhang, WH.; Ren, SD.; Bao, YP.; Qiao, YL. (Jan 2006). "Distribution of cervical intraepithelial neoplasia 2, 3 and cancer on the uterine cervix.". J Low Genit Tract Dis 10 (1): 45-50. PMID 16378031.
- ↑ V. Dube 2008.
- ↑ Singh, M.; Mockler, D.; Akalin, A.; Burke, S.; Shroyer, A.; Shroyer, KR. (Feb 2012). "Immunocytochemical colocalization of P16(INK4a) and Ki-67 predicts CIN2/3 and AIS/adenocarcinoma.". Cancer Cytopathol 120 (1): 26-34. doi:10.1002/cncy.20188. PMID 22162342.
- ↑ Jackson, JA.; Kapur, U.; Erşahin, Ç. (Apr 2012). "Utility of p16, Ki-67, and HPV test in diagnosis of cervical intraepithelial neoplasia and atrophy in women older than 50 years with 3- to 7-year follow-up.". Int J Surg Pathol 20 (2): 146-53. doi:10.1177/1066896911427703. PMID 22104735.
- ↑ del Pino, M.; Garcia, S.; Fusté, V.; Alonso, I.; Fusté, P.; Torné, A.; Ordi, J. (Nov 2009). "Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1.". Am J Obstet Gynecol 201 (5): 488.e1-7. doi:10.1016/j.ajog.2009.05.046. PMID 19683687.
- ↑ Nucci, Marisa R.; Oliva, Esther (2009). Gynecologic Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 167. ISBN 978-0443069208.
- ↑ URL: http://www.womenshealthsection.com/content/print.php3?title=gynpc006&cat=60&lng=english. Accessed on: 20 March 2013.
- ↑ De Boer, MA.; Peters, LA.; Aziz, MF.; Siregar, B.; Cornain, S.; Vrede, MA.; Jordanova, ES.; Fleuren, GJ. (Apr 2005). "Human papillomavirus type 18 variants: histopathology and E6/E7 polymorphisms in three countries.". Int J Cancer 114 (3): 422-5. doi:10.1002/ijc.20727. PMID 15551313.
- ↑ http://www.nature.com/modpathol/journal/v15/n3/pdf/3880520a.pdf
- ↑ 35.0 35.1 URL: http://sunnybrook.ca/content/?page=dept-labs-apath-gynpath-imgat-cvx-mal-microiscc. Accessed on: 2 May 2013.
- ↑ URL: http://missinglink.ucsf.edu/lm/IDS_107_Cervix_Ovary_Uterus/homepage.htm. Accessed on: 2 May 2013.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1077. ISBN 0-7216-0187-1.
- ↑ LAE. 15 January 2009.
- ↑ 39.0 39.1 39.2 Park, JJ.; Sun, D.; Quade, BJ.; Flynn, C.; Sheets, EE.; Yang, A.; McKeon, F.; Crum, CP. (Oct 2000). "Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia?". Am J Surg Pathol 24 (10): 1414-9. PMID 11023104.
- ↑ URL: http://sunnybrook.ca/content/?page=Dept_LabS_APath_GynPath_ImgAt_Cvx_mal_ais_smile. Accessed on: 30 March 2012.
- ↑ Togami, S.; Kasamatsu, T.; Sasajima, Y.; Onda, T.; Ishikawa, M.; Ikeda, S.; Kato, T.; Tsuda, H. (2012). "Serous adenocarcinoma of the uterine cervix: a clinicopathological study of 12 cases and a review of the literature.". Gynecol Obstet Invest 73 (1): 26-31. doi:10.1159/000329319. PMID 21876330.
- ↑ Kosińiska-Kaczyńska, K.; Mazanowska, N.; Bomba-Opoń, D.; Horosz, E.; Marczewska, M.; Wielgoś, M. (Dec 2011). "Glassy cell carcinoma of the cervix--a case report with review of the literature.". Ginekol Pol 82 (12): 936-9. PMID 22384631.
- ↑ van Dijck, JA.; Doorduijn, Y.; Bulten, JH.; Verloop, J.; Massuger, LF.; Kiemeney, BA. (2009). "[Vaginal and cervical cancer due to diethylstilbestrol (DES); end epidemic]". Ned Tijdschr Geneeskd 153: A366. PMID 19857300.
- ↑ 44.0 44.1 44.2 44.3 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 442. ISBN 978-0781765275.
- ↑ Babić, D.; Kos, M.; Jukić, S.; Ilić, J.; Vecek, N.; Kos, M.; Mahnik, N.. "[Clear cell carcinoma of the female genital tract].". Jugosl Ginekol Perinatol 31 (3-4): 102-4. PMID 1749271.
- ↑ Kocken, M.; Baalbergen, A.; Snijders, PJ.; Bulten, J.; Quint, WG.; Smedts, F.; Meijer, CJ.; Helmerhorst, TJ. (Aug 2011). "High-risk human papillomavirus seems not involved in DES-related and of limited importance in nonDES related clear-cell carcinoma of the cervix.". Gynecol Oncol 122 (2): 297-302. doi:10.1016/j.ygyno.2011.05.002. PMID 21620450.
- ↑ 47.0 47.1 Senzaki H, Osaki T, Uemura Y, et al. (December 1997). "Adenoid basal carcinoma of the uterine cervix: immunohistochemical study and literature review". Jpn. J. Clin. Oncol. 27 (6): 437–41. PMID 9438010. http://jjco.oxfordjournals.org/cgi/content/full/27/6/437.
- ↑ Nasu, K.; Takai, N.; Narahara, H. (Jun 2009). "Multimodal treatment for glassy cell carcinoma of the uterine cervix.". J Obstet Gynaecol Res 35 (3): 584-7. doi:10.1111/j.1447-0756.2008.00968.x. PMID 19527406.
- ↑ Kosińiska-Kaczyńska, K.; Mazanowska, N.; Bomba-Opoń, D.; Horosz, E.; Marczewska, M.; Wielgoś, M. (Dec 2011). "Glassy cell carcinoma of the cervix--a case report with review of the literature.". Ginekol Pol 82 (12): 936-9. PMID 22384631.
- ↑ Reis-Filho, JS.; Fillus Neto, J.; Schonemann, E.; Sanderson, A.; Schmitt, FC.. "Glassy cell carcinoma of the uterine cervix. Report of a case with cytohistologic and immunohistochemical study.". Acta Cytol 45 (3): 407-10. PMID 11393075.
- ↑ URL: http://www.webpathology.com/image.asp?n=2&Case=561. Accessed on: 4 September 2011.
- ↑ Deshpande, AH.; Kotwal, MN.; Bobhate, SK.. "Glassy cell carcinoma of the uterine cervix a rare histology. Report of three cases with a review of the literature.". Indian J Cancer 41 (2): 92-5. PMID 15318016.
- ↑ Fadare, O.; Zheng, W. (Nov 2005). "Well-differentiated papillary villoglandular adenocarcinoma of the uterine cervix with a focal high-grade component: is there a need for reassessment?". Virchows Arch 447 (5): 883-7. doi:10.1007/s00428-005-0030-3. PMID 16088403.
- ↑ Korach, J.; Machtinger, R.; Perri, T.; Vicus, D.; Segal, J.; Fridman, E.; Ben-Baruch, G. (2009). "Villoglandular papillary adenocarcinoma of the uterine cervix: a diagnostic challenge.". Acta Obstet Gynecol Scand 88 (3): 355-8. doi:10.1080/00016340902730359. PMID 19172445.
- ↑ Zaino, RJ.; Kurman, RJ.; Brunetto, VL.; Morrow, CP.; Bentley, RC.; Cappellari, JO.; Bitterman, P. (Nov 1998). "Villoglandular adenocarcinoma of the endometrium: a clinicopathologic study of 61 cases: a gynecologic oncology group study.". Am J Surg Pathol 22 (11): 1379-85. PMID 9808130.
- ↑ Nucci, Marisa R.; Oliva, Esther (2009). Gynecologic Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 180-1. ISBN 978-0443069208.
- ↑ 57.0 57.1 57.2 Lennerz, JK.; Perry, A.; Mills, JC.; Huettner, PC.; Pfeifer, JD. (Jun 2009). "Mucoepidermoid carcinoma of the cervix: another tumor with the t(11;19)-associated CRTC1-MAML2 gene fusion.". Am J Surg Pathol 33 (6): 835-43. doi:10.1097/PAS.0b013e318190cf5b. PMID 19092631.
- ↑ 58.0 58.1 58.2 Thelmo, WL.; Nicastri, AD.; Fruchter, R.; Spring, H.; DiMaio, T.; Boyce, J. (1990). "Mucoepidermoid carcinoma of uterine cervix stage IB. Long-term follow-up, histochemical and immunohistochemical study.". Int J Gynecol Pathol 9 (4): 316-24. PMID 1700969.
- ↑ URL: http://www.thefreedictionary.com/retiform. Accessed on: 25 August 2012.
- ↑ 60.0 60.1 60.2 Tsuda, H.; Mikami, Y.; Kaku, T.; Akiyama, F.; Hasegawa, T.; Okada, S.; Hayashi, I.; Kasamatsu, T. (Jul 2003). "Interobserver variation in the diagnosis of adenoma malignum (minimal deviation adenocarcinoma) of the uterine cervix.". Pathol Int 53 (7): 440-9. PMID 12828609.
- ↑ Lim, KT.; Lee, IH.; Kim, TJ.; Kwon, YS.; Jeong, JG.; Shin, SJ. (Mar 2012). "Adenoma malignum of the uterine cervix: Clinicopathologic analysis of 18 cases.". Kaohsiung J Med Sci 28 (3): 161-4. doi:10.1016/j.kjms.2011.10.009. PMID 22385609.
- ↑ Riegert-Johnson, D.; Roberts, M.; Gleeson, FC.; Krishna, M.; Boardman, L. (Sep 2011). "Case studies in the diagnosis and management of Peutz-Jeghers syndrome.". Fam Cancer 10 (3): 463-8. doi:10.1007/s10689-011-9438-x. PMID 21503748.
- ↑ Ito, M.; Minamiguchi, S.; Mikami, Y.; Ueda, Y.; Sekiyama, K.; Yamamoto, T.; Takakura, K. (Aug 2012). "Peutz-Jeghers syndrome-associated atypical mucinous proliferation of the uterine cervix: A case of minimal deviation adenocarcinoma ('adenoma malignum') in situ.". Pathol Res Pract. doi:10.1016/j.prp.2012.06.008. PMID 22878090.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 441-2. ISBN 978-0781765275.