Difference between revisions of "Neuromuscular pathology"
Jensflorian (talk | contribs) (→Wallerian degeneration: update) |
Jensflorian (talk | contribs) |
||
| Line 932: | Line 932: | ||
===Other Diseases=== | ===Other Diseases=== | ||
*Toxic polyneuropathy (drug toxicity).<ref>URL: [http://path.upmc.edu/cases/case173.html http://path.upmc.edu/cases/case173.html]. Accessed on: 8 January 2012.</ref> | *Toxic polyneuropathy (drug toxicity).<ref>URL: [http://path.upmc.edu/cases/case173.html http://path.upmc.edu/cases/case173.html]. Accessed on: 8 January 2012.</ref> | ||
*Polyglucosan body disease. | |||
===Neoplasms=== | ===Neoplasms=== | ||
Revision as of 12:45, 11 October 2019
Neuromuscular pathology is the study of muscle and neurologic disease associated with muscle dysfunction. It is a part of neuropathology.
Muscle pathology is dealt together with neurologic disease as, at the presentation, they are not infrequently impossible to definitely distinguish.
Work-up
General
- Clinical history, including family history.
- Laboratory studies, e.g. CK.
- Nerve conduction and electromyography studies.
- Muscle / nerve biopsy.
Clinical
- Fasciculations - small involuntary muscle contraction, imply lower motor neuron lesion.
- Reflexes - see physical examination.
- Strength.
Laboratory studies
The CK suggest the type of disorder:[1]
- High ~200-300X normal -- suggests myogenic.
- Intermedidate ~20-30X normal -- possibly inflammatory.
- Low ~2-5X normal -- possibly neurogenic.
Notes:
- The CK value is most useful when it is very high.[2]
- Normal CK values:[3]
- Men: 24-195 unit/litre.
- Women: 24-170 units/litre.
Biopsy
Muscle biopsies
Indications:
- Weakness, Fatigue, Cramps
- Myopathic EMG
- Elevated CK
Not indicated: Myasthenia gravis, myotonia
- MRI to select ideal spots for biopsy.
- In chronic diseases, select a moderately affected muscle.
- Best specific muscles: Deltoid, Biceps, Quadriceps.
- Avoid areas with previous EMG analysis.
- Tissue should be sent fresh or frozen for analysis.
- Freeze most tissue in isopentane (-160°C) immersed in liquid nitrogen.
- Ultrastructural analyis might be required in some cases -> save something in 4% glutaraldehyde.
- FFPE specimens unsuitable for enzymatic stains.
- Useful for morphology of inflammatory cells.
Nerve biopsies
- Nerve procession: 3 pieces
- Frozen -> useful for acid phosphatase, congo etc..
- Formalin -> for IHC.
- 4% Glutaraldehyde fixed -> for electron microscopy.
Skin biopsies
- Punch biopsies (3mm) for small fiber neuropathy.
- Paraformaldehyde-lysine-periodate -> for PGP9.5 immunofluorescence.
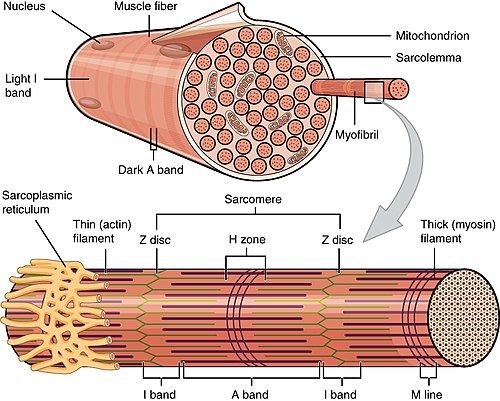
Muscle structure/histology
Macroscopic to microscopic
Organization:[4]
- Muscle - surrounded by epimysium.
- Fascicle - surrounded by perimysium.
- Muscle fibre - muscle cell.
- Myofibrils - contractile elements within the muscle cell.
- Muscle fibre - muscle cell.
- Fascicle - surrounded by perimysium.
Notes:
- This is similar for nerves:[5]
- Nerve (surrounded by epineurium) -> Fascicle (surrounded by perineurium) -> Nerve fibre (surrounded by endoneurium).
Fibre
Fibre morphology
- Small or large?
- Related to age? Birth 15µm, 6yrs: 25-30µm, 12yrs: 45µm, adult: 50-60µm.
- Round or angular?
- Architecture: Normal, inclusions, nuclear internalization?
- Pathology distribution: Absent, focal, uniform?
- Pathologic material: Amyloid, Glycogen, Lipid?
Fibre types
| Types | |||||||||||||||||||
| Type 1 slow twitch | Type 2 fast twitch | ||||||||||||||||||
Type 1 - AKA slow twitch:
- Predominantly oxidative metabolism, i.e. have lots of mitochondria.
Type 2 - AKA fast twitch:
- Predominantly glycolytic metabolism.
Mnemonic for type I fibres slow fat red ox:
- Slow twitch fibres are lipid rich (fat), (grossly) more red (due to mitochondria) and primarily have oxidative metabolism.
Table - fibre types
| Parameter | Type I | Type II |
|---|---|---|
| Twitch speed | slow | fast |
| Colour | red | white |
| Fat content | higher | lower |
| ATP production | oxidative | anaerobic glycoloysis |
| Glycogen | higher | lower |
| Resistance to fatigue | higher | lower |
| ATPase quality | lower | higher |
| Myoglobin | higher | lower |
| Mitochondria | higher | lower |
| ATPase pH 9.4 stain | light brown | dark brown |
- Check for fibre type grouping or fibre type predominance.
Normal findings
Muscle-tendon junction
Features:
- Myofibrils frayed + adjacent to dense connective tissue.
Muscle-nerve junction
Features:
- Dunno. (???)
Images:
Muscle spindle
Features:
- Weird looking muscle cell. (???)
Image: Muscle spindle (anhb.uwa.edu.au).[7]
Abnormal findings
Iatrogenic
- Torn (muscle) fibres (in the process of extraction for examination):
- Membrane intact.
- Myofibril kaputt.
- No inflammation.
Pathologic
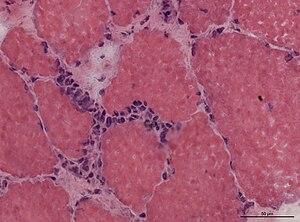
- Ragged red fibres = mitochondrial pathology.
- Image: Ragged red fibres (ouhsc.edu).
- Vacuoles
- Acid maltase positive = lysosomal vacuoles.
- Rimmed vacuoles = inclusion body myositis.
- Freezing artifacts (clear).
- PAS +++ = glycogen storage disease.
- Nemaline rods = nemaline myopathy
- Image: nemaline myopathy gomori.jpg
- Cores - central pale area along length of fibres in NADH stain = central core disease.
- Image: core disease NADH stain.jpg.
Others:
- Annular myofibrils ("ringbinden") = myopathic: Regeneration, myotonic dystrophy, tenotomy. Found in approx. 3% of unselected cases.
Images: [1] - HE, NADH or MAD stains are useful.
- Target fibre - "hole in middle of myofibres" = neurogenic.
- Regenerative fibres = large nuclei, basophilic cytoplasm (incr. protein synthesis, incr. RNA).
Approach
General:
- Neurogenic or myopathic?
- Acute or chronic?
Check:
- Size variation - in groups (neurogenic, Dystrophinopathies) vs. singular scattered (myogenic, acute neurogenic).
- Shape - angulated (neurogenic) vs. round (myogenic).
- Position of nuclei - peripheral (normal); central (myogenic; centronuclear myopathy[8]).
- Necrosis & regeneration - suggests acute myogenic.
- Fibrosis - suggests chronic myogenic.
- Inflammation - suggest myogenic vs. systemic inflammatory.
- Lymphocytes, macrophages, eosinophils - or even neoplastic?
- Fibre type predominance - suggest congenital myopathy (esp. in small type 1 fibres), demyelinating neuropathy.
Other:
- Obvious abnormality vs. minimal change.
- Diffuse vs. focal change.
- Pathology in adjacent vessels or connective tissue.
Processing of muscle biopsies
- Formalin fixed (formalin fixed-paraffin embedded).
- Frozen tissue for histology.
- Frozen tissue for biochemistry.
- Fragment for electron microscopy (glutaraldehyde fixed).
SMH labeling
- "E" = "frozens"; done on frozen tissue.
- IHC done on these.
- May have the label "2" ... even though there is no part 2.
- Blue slides = "plastics", i.e. plastic embedded.
- Stained with methylene blue.[9] vs. toluidine blue. (???)
- Thin sections: 0.1 - 1 micrometres.
- Normal SMH numbering = "paraffin".
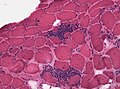
Patterns (pathologic)
Overview
| Neuromuscular pathology | |||||||||||||||||||||||||||||||||
| Neurogenic | Myogenic | Other/Mixed | |||||||||||||||||||||||||||||||
| Neurogenic | Myogenic | Notes | Image | |
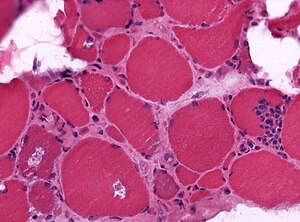
| Shape of fibres | angulated | round | round fibres[10] | |
| Small fibres | groups ("group atrophy") |
singular | group atrophy[11] | |
| Large fibres |
no | +/-scattered | "hypercontracted fibres" |
DMD (WC) |
| Fibre type grouping |
yes (d/t chronic denervation + reinnervation)[12] |
yes (???) | based on ATPase, NADH-TR stains |
ATPase 9.4[13], NADH-TR[14] |
List
Neurogenic:
- Angulated myocytes.
- Groups of small fibres.
- Apparent increase of nuclei.
Myogenic:
- Round myocytes.
- +/-Intense (darker) cytoplasm.
- +/-Fibrosis (between fibres).
- +/-Nuclear internalization.
- +/-Necrosis.
Detail
- Segmental demyelination - nerve/CNS abnormality.
- Axonal degeneration - nerve/CNS abnormality.
- Reinnervation - nerve injury.
- Myopathy - something is wrong with the muscle fibres.
Stains for muscle biopsies
Standard
| Stain | Comment | Image |
| H&E stain | routine, fibre size, shape, nuclei | H&E[15], H&E (WC) |
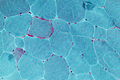
| Gomori trichrome | good for nemaline rods, mitochondrial pathology (ragged red fibres - at edge of myocyte) |
RRF (WC) |
| PAS | glycogen storage disorders | [2][16] |
| Congo red | find amyloid; seen in inclusion body myositis |
[3][17] |
| Oil red O | lipid more in type 1 fibres |
ORO |
| ATPase pH4.2 ATPase pH9.4 |
should have "checkerboard pattern" in normal; see table below |
[4][16] |
| NADH-TR | good for cores or tubular aggregates, should have "checkerboard pattern" in normal; type 1 fibres = light blue, type 2 fibres = white |
[5] |
| Myoadenylate deaminase | Normal: positive, AMPDA deficiency: negative | MAD deficiency |
| Acid phosphatase | Histiocytes/Macrophages, Lysosomal storage, Lipofuscin | |
| Cytochrome oxidase | Mitochondrial pathology | COX deficiency |
ATPase stain pattern/fibre type
| Type 1 slow twitch |
Type 2 fast twitch | |
| pH 4.2 | dark | light |
| pH 9.4 | light | dark |
Special - mitochondrial pathology
| Stain | Comment | Image |
| Succinate dehydrogenase (SDH) |
stains mitochondria; usu. +ve in mitochondrial disease[18] |
[6][19], SDH (WC) |
| Cytochrome oxidase (COX) | stains mitochondria; usu. -ve in mitochondrial disease |
[7][19] |
| COX-SDH | used to look for mitochondrial disease |
Enzymatic/genetic stuff
| Stain | Comment | Image |
| Phosphorylase | ||
| Adenylate deaminase | ||
| Acid phosphatase (ACPH) | necrosis (red) | |
| Alkaline phosphatase (ALPH) | regeneration (punctate - black) |
Dunno:
- Toluidine blue - myopathies.
- Image: Nemaline rods (wustl.edu).[20]
IHC
- Dystrophy panel.
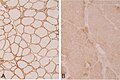
- Dystrophin[21] - Duchenne muscular dystrophy (onset usu. <3 years), Becker's muscular dystrophy (onset usu. 20s or 30s).
- Membranous staining is normal. Loss of membranous staining = pathologic.
- Tested with three antibodies -- as the protein is hueuge.
- Membranous staining is normal. Loss of membranous staining = pathologic.
- Spectrin - a cause of long QT syndrome. (???)
- Dystrophin[21] - Duchenne muscular dystrophy (onset usu. <3 years), Becker's muscular dystrophy (onset usu. 20s or 30s).
- Lymphocytic markers (CD45, CD3, CD4, CD8, CD20).
- MAC - inclusion body myositis.
- APP - inclusion body myositis (?), axonal swellings.
- Ubquitin - inclusion body myositis.
- TDP-43 (also TDP43) - cytoplasmic staining in IBM.
- Normally stains the nucleus.
- Same protein that that in implicated in ALS and frontotemporal dementia.
- Normally stains the nucleus.
List of common conditions
Neurogenic:
- Amyotrophic lateral sclerosis.
- Spinal muscular atrophy.
- Trauma.
- Vascular disease.
- Infective process.
- ?Motor neuron disease.
Myopathic:
- Inflammatory:
- Duchenne muscular dystrophy.
- Becker muscular dystrophy.
- Limb-girdle muscular dystrophy.
- Myotonic dystrophy.
- Metabolic - glycogen storage disease.
Other:
- Myasthenia gravis.
- Mitochondrial myopathy.
- Congenital fibre type disproportion.
- Periodic paralysis.
Groups of disorders
Inflammatory myopathy
DDx:
- Polymyositis.
- Disease of adults.
- Inclusion body myositis (IBM).
- Distal weakness.
- Can be sporadic or hereditary.
- Dermatomyositis.
- Acute development.
- May be associated with malignancy.
- Granulomatous myositis.
- Graft-versus-host disease.
- Infectious myositis.
- Rare.
Quick overview:
| Dermatomyositis | Polymyositis | sporadic Inclusion body myositis | |
| Myositis type | Perifascicular | Diffuse | Diffuse (limited inflammation) |
| Histology | Perivascular inflammation, Perifascicular damage. | Endomysial inflammation and damage. | Endomysial inflammation, rimmed vacuoles withe eosinophilic inclusions, neurogenic changes. |
| Immunostaining | CD4+ B-cell lymphocytes predominate, C5b9 complement complex deposits in capillaries. | CD8+ lymphocytes invading non-necrotic fibers. | Mainly CD8-positive lymphocytes. |
| Electron microscopy | Tubulovesicular inclusions. | Nothing special. | Filamentous inclusions. |
| Exemplary image |
Partial invasion of muscle fibres
DDx:[22]
- Polymyositis.
- Inclusion body myositis (IBM).
Images:
Muscular dystrophy
General
- DDx: large.
A short DDx:
- Duchenne's muscular dystrophy.[21]
- Becker's muscular dystrophy.
- Limb-girdle muscular dystrophy.
- Lotsa different mutations, autosomal dominant and recessive variants.
- Myotonic dystrophy.[23][24]
Microscopic
Features:
- Endomysial fibrosis.
- Hypercontracted fibres (large muscle fibres).
Images:
Limb-girdle muscular dystrophy
General
- A group of muscular dystrophies with childhood or adult onset.[25]
- Rare.
- Usually autosomal recessive.
- Treatment: none; supportive only.
Subtypes
- Sarcoglycanopathy.
- Calpainopahty.
- Dysferlinopathy.
Notes:
- Can be demonstrated with IHC.
DDx
- DMD gene associated MDs (Duchenne MD, Becker MD).
- Facioscapulohumeral muscular dystrophy (FSHD).
- Emery-Dreifuss MD (EDMD).
- Congenital MD (CMD).
- Inflammatory myopathies.
Mitochondrial disorders
General
- Onset childhood to adulthood.
- Heteroplasmy - variable distribution of badness within affected individuals.
- Leads to "threshold effect".
Microscopic
- Trichrome most useful - find the ragged red fibres - usu. at the cell periphery.
- COX-SDH:
- Non-staining (???).
- Peripheral blue accumulation in occasional cells.
EM
Features:
- Crystalloid inclusions.[26]
- "Ballooned" mitochondria; loss of cristae -- loss of membranous folds within mitochrondrion.
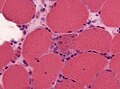
Type 2 fibre atrophy
General
DDx:
- Disuse.
- Space travel.
- Steroids.
- Others.
Microscopic
Features:
- Atrophy for type 2 atrophy.
Images
Specific entities
Amyotrophic lateral sclerosis
- Abbreviated ALS.
General
- Abbreviated ALS.
- Affects - corticospinal tract - gliosis.
Microscopic
Features:
- Neurogenic pattern:
- Group atrophy.
- +/-Target fibres.
Dermatomyositis
- For the skin manifestations see: Inflammatory_skin_disorders#Dermatomyositis.
General
- Complement mediated disease - membrane attack complex.
- Usually middle age.
- Associated skin rash is common.
- May precede or follow muscle pathology.
- Associated with malignancy in approximately 10% of cases.[27]
Clinical
- If the characteristic skin lesions are absent... it is likely idiopathic inflammatory myositis and related to diabetes mellitus.[28]
Microscopic
Features:
- Perifascicular inflammation with perifascicular atrophy - key feature.
- Loss of vessels around muscle fibres.
- Vessels should be where more than 3 or more fibres are opposed to one another.
Images
EM
- Endothelial tubuloreticular inclusions (abbrev. TRIs) - undulating tubules in the smooth ER, usu. perinuclear;[29] not pathognomonic - may be seen in inclusion body myositis.[30]
Images:
DDx:
- Anti-Jo1 myositis
- Paraneoplastic myositis
Inclusion body myositis
General
- Usually elderly.
- Thought to be related to Alzheimer's disease due to similar staining with congo red and several IHC stains.[31]
Microscopic
Features:
- Inflammation.
- Vacuolated muscle fibres (with proteineous aggregates) - key feature.
- Vacuolation = "inclusion".
- Usually in the centroidal location.
- Vacuolation = "inclusion".
DDx:
IHC
Features:[31]
- Congo red +ve.
- APP +ve, ubiquitin +ve, tau +ve. (???)
EM
- Inclusion bodies - tubulovescicular material.[32]
Polymyositis
General
- Tx: steroids.
Microscopic
Features:[33]
- Lymphocytes - may be in large clusters.
- "Partial invasion" - lymphocytes within the muscle fibres - key feature.
- Regenerating fibres with enlarged nuclei.
DDx:
Images:
IHC
Features:[33]
- T cells > B cells.
- Endomysial - T cells.
- Perimysial - B cells.
Granulomatous myositis
General
Etiology:[34]
- Sarcoidosis
- Idiopathic
- Infectious (Tuberculosis, Syphillis, Brucellosis
- Foreign-body reaction
- Thymoma - myasthenia gravis
- Lymphoma - paraneoplastic
- Primary biliary cirrhosis
Spinal muscular atrophy
- Autosomal recessive disease by SMN1 gene deletion on chromosome 5q.
- Centromeric gene copy (SMN2) whose product can mitigate disease severity.
- Variability in severity and age of onset of disease (SMA type 1-4).
- Neurogenic muscle atrophy, weakness, loss of reflexes, tongue fasciculation and tremor.
- Usu. groups of atrophic fibers.
- Few compensatorirc hypertrophic fibers.
Diagnostic relevance
- Antisense-oligonucleotide that increase full-length protein product derived from SMN2 (Nusinersen).
- Gene transfer with scAAV9-SMN (Zolgensma).
Metabolic myopathy
Microscopic
Fetures:
- Intramuscular storage deposits.
- PAS positive stain in glycogen storage disease.
Myotonic dystrophy
Microscopic
Features:
- Internal nuclei/central nuclei.
Nemaline myopathy
General
- A type of congenital myopathy.
- Paediatric thingy.
- May appear secondary in other lesions.
- Rods are seen in trichrome stain
Central core myopathy
General
- Floppy infant, but stable clinial course.
- Autosomal dominant inheritance.
- Mutation in RYR1
- Predisposition for malignant hyperthermia.
- Normal CK levels.
- Non-pathologic EMG.
- Cores visile in NADH staining.
- Mostly centrally, but can be eccentric.
Centronuclear myopathy
- AKA myotubular myopathy
- Several types
- X-chromosomal recessive: floppy infant
- austosmal dominant: late onset with proximal involvement, ptosis, opthalmoplegia
Image centronuclear myopathy[35]).
Drug-induced rhabdomyolysis
- AKA drug-induced acute necrotizing myopathy.
General
Clinical:[36]
- Myalgias.
- Myoglobinuria.
- Increased elevated serum creatine kinase (CK).
Causes:
- Ecstasy (MDMA).
- Statins.
Microscopic
Features:
- Muscle necrosis.
- No inflammation.
- No perifascicular atrophy.
Images:
Stains
- PAS +ve fibres (macrophages).
IHC
- CD45 -ve (no lymphocytes).
EM
- Negative for tubuloreticular inclusions.
Trichinosis
- See Microorganisms.
Parasitic disease classically associated with consumption of uncooked pork.
Nerve stuff
General
- Most common biopsy: sural nerve.
- Approx. 20-30% of the biopsies are diagnostic or may alter treatment decisions.
- Far less common: Superficial peroneal nerve.
- Metabolic, toxic and nutritional causes account for 50% of neuropathies.
- Inflammatory neuropathies (mostly GBS, CIDP or vasculitis): 10-20%.
- Familial neuropathy: 10-20%.
- Neoplasm-associated neuropathy: 5-10%.
Nerve structure
- Nerve (surrounded by epineurium).
- Fascicle (surrounded by perineurium).
- Usu 6-15 fascicles in sural nerve.
- Nerve fibre (surrounded by endoneurium).
- Myelinated axons.
- Unmyelinated axons and their Schwann cells together are called Remak bundles.
Epineurium:
- Capillaries, arterioles and venules.
- Fibroblasts (CD34+/-ve, EMA-ve, S100-ve).
- Macrophages (CD68+ve, CD168+ve).
- Mast cells (metachromatic granules).
- Leukocytes (usu. less than 10 CD3+ve Lymphocytes/mm²).
- Pacinian corpuscles (no pathological relevance).
Perineurium:
- Fascicles may separated by perineurial septae.
- Occasional perineurial calcifications (no pathological relevance).
- Renaut bodies (subperineurial whorled structures consisting of fibroblasts).
Stains
Myelin stain:
- Blue = myelin.
Gomori trichrome:
- Axon = green.
- Myelin = red.
Reactive changes
- Traumatic Neuroma
- Pacinian Neuroma
- Nerve cysts.
- Neuritis ossificans.
- Localized interdigital neuritis (Morton neuroma).
Degenerative changes
Types:[37]
- Axonal degeneration.
- Wallerian degeneration.
- Segmental demyelination.
Axonal degeneration
- Axonal swelling.
- Intra-axonal filamentous aggregates.
- Mitochondrial abnormalities.
- Aggregation of organelles and dense bodies.
Wallerian degeneration
- Watery axon and granular disintegration (distal).
- Macrophage accumulation (3-4d after transsection).
- Many lysosomes (CD68+ve).
- Endoneurial proliferation.
- Digestion chambers - key feature.
Images:
Segmental demyelination
- Onion bulb formations - key feature.
Inflammation
- Inflammatory pseudotumour.
- Leprosy (Leprous neuropathy).
- Sarcoidosis.
- Neuropathy with macrophage-induced demyelination (CIDP, GBS).
- Guillain–Barré syndrome.
- Chronic inflammatory demyelinating polyneuropathy (CIDP).[38]
- CMV neuritis in immuncompromised patients.
- Vasculitis.
Other Diseases
- Toxic polyneuropathy (drug toxicity).[39]
- Polyglucosan body disease.
Neoplasms
Main article: Peripheral nerve sheath tumours
- Nerve sheath tumors:
- Non neurogenic-tumors of the nerve:
See also
References
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/NeuroHelp/ZNEWWU10.htm. Accessed on: 27 October 2010.
- ↑ Filosto M, Tonin P, Vattemi G, et al. (January 2007). "The role of muscle biopsy in investigating isolated muscle pain". Neurology 68 (3): 181–6. doi:10.1212/01.wnl.0000252252.29532.cc. PMID 17224570.
- ↑ URL: http://www.gpnotebook.co.uk/simplepage.cfm?ID=1436155929. Accessed on: 27 October 2010.
- ↑ URL: http://commons.wikimedia.org/wiki/File:Skeletal_muscle.jpg. Accessed on: 25 October 2010.
- ↑ Martini, Frederic H. (2003). Fundamentals of Anatomy & Physiology (6th ed.). Benjamin Cummings. pp. 438. ISBN 978-0805359336.
- ↑ URL: http://www.lab.anhb.uwa.edu.au/mb140/corepages/connective/connect.htm. Accessed on: 4 November 2010.
- ↑ URL: http://www.lab.anhb.uwa.edu.au/mb140/corepages/muscle/muscle.htm. Accessed on: 28 November 2010.
- ↑ URL: http://www.igbmc.fr/recherche/Dep_NG/Eq_JLaporte/JL3.html. Accessed on: 26 October 2010.
- ↑ URL: http://www.nature.com/modpathol/journal/v18/n5/full/3800344a.html. Accessed on: 26 November 2010.
- ↑ URL: http://nmdinfo.org/lectures/info.php?id=8. Accessed on: 25 October 2010.
- ↑ URL: http://neuropathology.neoucom.edu/chapter9/chapter9fALS.html. Accessed on: 25 October 2010.
- ↑ URL: http://neuromuscular.wustl.edu/lab/mbiopsy.htm#fibertype. Accessed on: 26 October 2010.
- ↑ URL: http://missinglink.ucsf.edu/lm/ids_104_musclenerve_path/student_musclenerve/musclepath.html. Accessed on: 26 October 2010.
- ↑ URL: http://moon.ouhsc.edu/kfung/JTY1/Com04/Com401-3-Diss.htm. Accessed on: 28 October 2010.
- ↑ URL: http://www.rvc.ac.uk/Research/Labs/NeuroLab/MuscleBiopsy.cfm. Accessed on: 26 October 2010.
- ↑ 16.0 16.1 URL: http://neuromuscular.wustl.edu/pathol/dermmyo.htm. Accessed on: 26 October 2010.
- ↑ URL: http://neuromuscular.wustl.edu/pathol/ibmpaget.htm. Accessed on: 26 October 2010.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/neurohelp/ZNEWWU10.htm. Accessed on: 2 March 2011.
- ↑ 19.0 19.1 URL: http://moon.ouhsc.edu/kfung/JTY1/Com04/Com401-3-Diss.htm. Accessed on: 28 October 2010.
- ↑ URL: http://neuromuscular.wustl.edu/pathol/rod.htm. Accessed on: 26 October 2010.
- ↑ 21.0 21.1 Online 'Mendelian Inheritance in Man' (OMIM) 310200
- ↑ 22.0 22.1 URL: http://neuromuscular.wustl.edu/pathol/inflammation.htm#cellinv. Accessed on: 3 November 2010.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 160900
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 602668
- ↑ URL: http://www.ncbi.nlm.nih.gov/books/NBK1408/. Accessed on: 25 November 2010.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/neurotest/Q09-Ans.htm. Accessed on: 26 October 2010.
- ↑ Chen YJ, Wu CY, Huang YL, Wang CB, Shen JL, Chang YT (2010). "Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan". Arthritis Res. Ther. 12 (2): R70. doi:10.1186/ar2987. PMC 2888225. PMID 20398365. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2888225/.
- ↑ Limaye VS, Lester S, Blumbergs P, Roberts-Thomson PJ (May 2010). "Idiopathic inflammatory myositis is associated with a high incidence of hypertension and diabetes mellitus". Int J Rheum Dis 13 (2): 132–7. doi:10.1111/j.1756-185X.2010.01470.x. PMID 20536597.
- ↑ Stoltenburg-Didinger G, Genth E (June 2009). "[Dermatomyositis]" (in German). Z Rheumatol 68 (4): 287–94. doi:10.1007/s00393-008-0398-y. PMID 19330338.
- ↑ Katzberg HD, Munoz DG (2010). "Tubuloreticular inclusions in inclusion body myositis". Clin. Neuropathol. 29 (4): 262–6. PMID 20569678.
- ↑ 31.0 31.1 Askanas V, Engel WK (November 1995). "New advances in the understanding of sporadic inclusion-body myositis and hereditary inclusion-body myopathies". Curr Opin Rheumatol 7 (6): 486–96. PMID 8579968.
- ↑ URL: http://neuromuscular.wustl.edu/pathol/ibm.htm. Accessed on: 3 November 2010.
- ↑ 33.0 33.1 URL: http://moon.ouhsc.edu/kfung/jty1/neurohelp/ZNN0TA01.htm. Accessed on: 25 February 2012.
- ↑ Prieto-González, S.; Grau, JM.. "Diagnosis and classification of granulomatous myositis.". Autoimmun Rev 13 (4-5): 372-4. doi:10.1016/j.autrev.2014.01.017. PMID 24424169.
- ↑ URL: http://www.igbmc.fr/recherche/Dep_NG/Eq_JLaporte/JL3.html. Accessed on: 26 October 2010.
- ↑ Coco, TJ.; Klasner, AE. (Apr 2004). "Drug-induced rhabdomyolysis.". Curr Opin Pediatr 16 (2): 206-10. PMID 15021204.
- ↑ URL: http://missinglink.ucsf.edu/lm/ids_104_musclenerve_path/student_musclenerve/nervepath.html. Accessed on: 9 November 2010.
- ↑ URL: http://path.upmc.edu/cases/case426.html. Accessed on: 14 November 2010.
- ↑ URL: http://path.upmc.edu/cases/case173.html. Accessed on: 8 January 2012.
- ↑ Gläsker, S.; Berlis, A.; Pagenstecher, A.; Vougioukas, VI.; Van Velthoven, V. (Mar 2005). "Characterization of hemangioblastomas of spinal nerves.". Neurosurgery 56 (3): 503-9; discussion 503-9. PMID 15730575.
- ↑ Scheithauer, BW.; Amrami, KK.; Folpe, AL.; Silva, AI.; Edgar, MA.; Woodruff, JM.; Levi, AD.; Spinner, RJ. (Apr 2011). "Synovial sarcoma of nerve.". Hum Pathol 42 (4): 568-77. doi:10.1016/j.humpath.2010.08.019. PMID 21295819.