Difference between revisions of "Neuropathology"
m (vauthors) |
|||
| (52 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
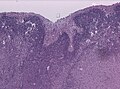
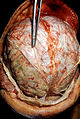
[[Image:MCA-Stroke-Brain-Human-2.JPG|thumb|right|Gross image showing changes of a [[stroke]]. (WC/Marvin 101)]] | |||
This article is an introduction to '''neuropathology'''. There are separate articles for [[brain tumours]], the [[pituitary gland]], the [[spine]], the [[eye]], [[muscle pathologies]], [[neurohistology]] and [[neuroanatomy]]. | This article is an introduction to '''neuropathology'''. There are separate articles for [[brain tumours]], the [[pituitary gland]], the [[spine]], the [[eye]], [[muscle pathologies]], [[neurohistology]] and [[neuroanatomy]]. | ||
| Line 8: | Line 9: | ||
==Neuroradiology== | ==Neuroradiology== | ||
Enhancing vs. non-enhancing: | Key factors to consider in evaluation: | ||
* | # Location. | ||
# Number of lesions - single versus multiple. | |||
# Cystic versus solid lesion. | |||
# Enhancement. | |||
==Lesion location== | |||
In neuroradiology and neuropathology, real estate is crucial. Lesion location can often narrow your differential. | |||
Cortical lesions (gray matter): | |||
* [[Oligodendroglioma]]. | |||
* [[DNET]]. | |||
* [[Ganglioglioma]]. | |||
* [[Pleomorphic xanthoastrocytoma]]. | |||
* Extraventricular [[ependymoma]]. | |||
Cortical-subcortical junction: | |||
* [[brain metastasis|Metastases]]. | |||
* Abscesses (hematogenous spread). | |||
Subcortical lesions (white matter): | |||
* [[Glioblastoma]]. | |||
* Diffuse gliomas. | |||
* Demyelinating plaques. | |||
Deep gray matter lesions (e.g. basal ganglia): | |||
* Gliomas. | |||
* [[Hypertensive hemorrhage]] | |||
Cerebellar lesions: | |||
* [[Medulloblastoma]]. | |||
* [[Pilocytic astrocytoma]]. | |||
* [[AT/RT]]. | |||
Intraventricular lesions: | |||
* [[Ependymoma]]. | |||
* [[Subependymoma]]. | |||
* [[Pilocytic astrocytoma]]. | |||
* [[Central neurocytoma]]. | |||
* Rosette forming glioneuronal tumour of the fourth ventricle. | |||
Suprasellar (above the pituitary): | |||
* [[Craniopharyngioma]]. | |||
* [[Germinoma]]. | |||
* [[Pilomyxoid astrocytoma]]. | |||
==Number of lesions== | |||
If ''single'' lesion = think primary, neoplastic | |||
If ''multiple'' lesions = think metastatic, neoplastic or infectious | |||
'''NB: glioblastoma can be multifocal''' (and the foci can be quite far apart) | |||
==Cystic vs. solid lesions== | |||
Some tumours are classically cystic with a small solid component (so-called cyst with a mural nodule) -- e.g. pilocytic astrocytoma, ganglioglioma, hemangioblastoma | |||
==Enhancing vs. non-enhancing:== | |||
*In adults, enhancing generally = high grade. | |||
*In pediatrics, it often depends on the pattern. | |||
Two main patterns to be mindful of -- ring enhancing lesions, and cystic lesions with a mural nodule. | |||
===Ring enhancing lesions=== | ===Ring enhancing lesions=== | ||
| Line 25: | Line 82: | ||
*Demyelination (e.g. [[multiple sclerosis]]). | *Demyelination (e.g. [[multiple sclerosis]]). | ||
*Resolving hematoma. | *Resolving hematoma. | ||
===Cyst with enhancing mural nodule=== | |||
*hemangioblastoma (#1 in adults) | |||
*pilocytic astrocytoma (#1 in peds) | |||
*pleomorphic xanthoastrocytoma | |||
*ganglioglioma | |||
==Grossing== | ==Grossing== | ||
| Line 52: | Line 115: | ||
<gallery> | <gallery> | ||
Image:Alzheimer_type_II_astrocyte_high_mag_cropped.jpg | Anoxic neurons (WC) | Image:Alzheimer_type_II_astrocyte_high_mag_cropped.jpg | Anoxic neurons (WC) | ||
Image:AcuteStroke_HE400x.jpg | Neurons in an acute stroke. (WC) | |||
</gallery> | </gallery> | ||
www: | www: | ||
*[http:// | *[http://neuropathology-web.org/chapter2/images2/2-anoxic.png Anoxic neurons (neuropathologyweb.org)].<ref>URL: [http://neuropathology-web.org/chapter2/chapter2aHIE.html http://neuropathology-web.org/chapter2/chapter2aHIE.html]. Accessed on: 10 December 2014.</ref> | ||
*[http://moon.ouhsc.edu/kfung/iacp-olp/APAQ-Images/N1-MS-01-16.gif Anoxic neurons (ouhsc.edu)].<ref>URL: [http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/N1-MS-01-16-Ans.htm http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/N1-MS-01-16-Ans.htm] and [http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/n1-ms-01.htm http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/n1-ms-01.htm]. Accessed on: 31 October 2010.</ref> | *[http://moon.ouhsc.edu/kfung/iacp-olp/APAQ-Images/N1-MS-01-16.gif Anoxic neurons (ouhsc.edu)].<ref>URL: [http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/N1-MS-01-16-Ans.htm http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/N1-MS-01-16-Ans.htm] and [http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/n1-ms-01.htm http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/n1-ms-01.htm]. Accessed on: 31 October 2010.</ref> | ||
| Line 154: | Line 218: | ||
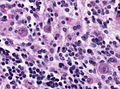
====Encephalitis==== | ====Encephalitis==== | ||
see also: | |||
* [[Viruses]] | |||
* [[Microorganisms]] | |||
=====General===== | =====General===== | ||
DDx: | DDx: | ||
*Viral encephalitis. | *Viral encephalitis (Neurotrophic viruses):<ref>{{Cite journal | last1 = Ludlow | first1 = M. | last2 = Kortekaas | first2 = J. | last3 = Herden | first3 = C. | last4 = Hoffmann | first4 = B. | last5 = Tappe | first5 = D. | last6 = Trebst | first6 = C. | last7 = Griffin | first7 = DE. | last8 = Brindle | first8 = HE. | last9 = Solomon | first9 = T. | title = Neurotropic virus infections as the cause of immediate and delayed neuropathology. | journal = Acta Neuropathol | volume = 131 | issue = 2 | pages = 159-84 | month = Feb | year = 2016 | doi = 10.1007/s00401-015-1511-3 | PMID = 26659576 }}</ref> | ||
** Eteroviruses are the most common cause of aseptic meningitis. | |||
***Coxackie Virus. | |||
***Enteric cytopathic human orphan (ECHO) virus. | |||
** Human Herpesviruses (HSV1, HSV2, VZV, CMV, EBV, Roseola) | |||
*** HSV encephalitis has high mortality without acyclovir treatment. | |||
*** Childhood cerebellitis mainly associated with varicella. | |||
*** VZV is the second most common viral meningitis after enterovirus. | |||
** Measles virus(worldwide more than 100.000 deaths annually). | |||
***Is linked to [[acute demyelinating encephalomyelitis]] (ADEM) and Subacute sclerosing encephalitis (SSPE). | |||
** Seasonal influenza A virus (highest patogenic potential: avian influenza H5N1). | |||
** Polio and Non-Polio Enterovirus (mostly children). | |||
***Although massive eradication: Polio still existent in Pakistan, Afghanistan, Nigeria. | |||
** Rabies virus | |||
** Tick-borne encephalitis virus (Europe, Siberia, Russian far-east). | |||
** West-Nile virus (US, Southern europe). | |||
** St. Louis encephalitis virus (US). | |||
** Japanese encephalitis virus (South, south-east asia, high disability rate). | |||
** La Crosse virus (esp. children, midwest & eastern US). | |||
** Borna disease virus (VSBV-1). | |||
** Equine encephalitis viruses (EEEV, VEEV, WEEV, CHIKV). | |||
*Paraneoplastic syndromes. | *Paraneoplastic syndromes. | ||
*Autoimmune antibody-mediated limbic encephalitis (NMDAR). | |||
*Purulent bacterial encephalitis | |||
**Streptococcus, [[Actinomyces]] .... | |||
*Septic metastatic encephalitis | |||
**microabscesses, local astrogliosis, two or more granulocytic infiltrates without relation to vessel.<ref>{{Cite journal | last1 = Tauber | first1 = SC. | last2 = Bunkowski | first2 = S. | last3 = Brück | first3 = W. | last4 = Nau | first4 = R. | title = Septic metastatic encephalitis: coexistence of brain damage and repair. | journal = Neuropathol Appl Neurobiol | volume = 37 | issue = 7 | pages = 768-76 | month = Dec | year = 2011 | doi = 10.1111/j.1365-2990.2011.01196.x | PMID = 21696418 }}</ref> | |||
*Septic embolic encephalitis | |||
**Embolic endocarditis, Stroke-like lesions.<ref>{{Cite journal | last1 = Bitsch | first1 = A. | last2 = Nau | first2 = R. | last3 = Hilgers | first3 = RA. | last4 = Verheggen | first4 = R. | last5 = Werner | first5 = G. | last6 = Prange | first6 = HW. | title = Focal neurologic deficits in infective endocarditis and other septic diseases. | journal = Acta Neurol Scand | volume = 94 | issue = 4 | pages = 279-86 | month = Oct | year = 1996 | doi = | PMID = 8937541 }}</ref> | |||
*Non-purulent bacterial encephalitis | |||
** [[Tuberculosis]]... | |||
=====Gross===== | =====Gross===== | ||
| Line 182: | Line 279: | ||
*Parasites, e.g. [[toxoplasma]]. | *Parasites, e.g. [[toxoplasma]]. | ||
*[[Fungi]], e.g. PASD. | *[[Fungi]], e.g. PASD. | ||
<gallery> | |||
File:CNS_lymphocytic_encephalitis_frozen_section.jpg | Intraoperative appearance of a lymphocytic encephalitis (WC/jensflorian) | |||
File:CNS_lymphocytic_encephalitis_FFPE_section.jpg | Perivascular inflammation in a lymphocytic encephalitis (WC/jensflorian) | |||
File:HSV_hemorrhagic_encephalitis.jpg | Hemorrhage in HSV encephalitis (WC/jensflorian) | |||
File:HSV_necrotizing_encephalitis.jpg | HSV encephalitis, higher magnification (WC/jensflorian) | |||
File:Cmv_status_verrucosus.jpg | Neonatal brain with migration disturbances due to CMV infection (WC/jensflorian) | |||
File:Cmv_neuronal_inclusions.jpg | Neuronal nuclear inclusions in a neonatal CMV infection (WC/jensflorian) | |||
File:Rabies encephalitis PHIL 3368 lores.jpg | Rabies encephalitis (CDC.gov) | |||
File:Rabies negri bodies brain.jpg | Negri bodies in Purkinje cells (CDC.gov) | |||
</gallery> | |||
====Vasculitis==== | |||
DDx Cerebral vasculitis / angiitis: | |||
*[[Systemic lupus erythematosus]] (SLE) | |||
*[[Rheumatoid arthritis]]. | |||
*Medications and drugs (amphetamine, cocaine and heroin). | |||
*Paraneoplastic(lymphomas, leukemia and lung cancer). | |||
*[[Granulomatosis_with_polyangiitis]] | |||
*[[Giant cell arteritis]] | |||
*[[Takayasu's arteritis]] | |||
*[[Polyarteritis nodosa]] | |||
*Beta-amyloid-related angiitis (ABRA) | |||
<gallery> | |||
File:ABRA_HE_40x.jpg | Beta-amyloid related angitis, HE (WC) | |||
File:ABRA_beta-amyloid_40x.jpg | Beta-amyloid related angiitis, abeta IHC (WC) | |||
File:Giant cell arteritis -- low mag.jpg | Giant cell arteritis, HE (WC) | |||
</gallery> | |||
===Architecture=== | ===Architecture=== | ||
| Line 197: | Line 323: | ||
***Image: [http://moon.ouhsc.edu/kfung/IACP-OLP/APAQ-Images/N1-TU-01-17.gif Medulloblastoma (ouhsc.edu)].<ref>URL: [http://moon.ouhsc.edu/kfung/IACP-OLP/APAQ-Text/N1-TU-01.htm#17 http://moon.ouhsc.edu/kfung/IACP-OLP/APAQ-Text/N1-TU-01.htm#17]. Accessed on: 3 December 2010.</ref> | ***Image: [http://moon.ouhsc.edu/kfung/IACP-OLP/APAQ-Images/N1-TU-01-17.gif Medulloblastoma (ouhsc.edu)].<ref>URL: [http://moon.ouhsc.edu/kfung/IACP-OLP/APAQ-Text/N1-TU-01.htm#17 http://moon.ouhsc.edu/kfung/IACP-OLP/APAQ-Text/N1-TU-01.htm#17]. Accessed on: 3 December 2010.</ref> | ||
**PNET (can be thought of as a supratentorial medulloblastoma) . | **PNET (can be thought of as a supratentorial medulloblastoma) . | ||
**Neuroblastoma | |||
*Flexner-Wintersteiner rosette = rosette with empty centre (donut hole).<ref name=pmid16551982/> | *Flexner-Wintersteiner rosette = rosette with empty centre (donut hole).<ref name=pmid16551982/> | ||
**[[Retinoblastoma]]s. | **[[Retinoblastoma]]s. | ||
**Pineoblastomas. | **[[Pineoblastomas]]. | ||
** | **[[Medulloepithelioma]]s. | ||
*True ependymal rosette = surrounds a space.<ref name=pmid16551982/> | *True ependymal rosette = surrounds a space.<ref name=pmid16551982/> | ||
| Line 210: | Line 337: | ||
**[[Pineocytoma]]. | **[[Pineocytoma]]. | ||
**[[Neurocytoma]]. | **[[Neurocytoma]]. | ||
**[[RGNT]] - Rosette forming glioneuronal tumor of the IVth ventricle. | |||
*Radial (cartwheel) profiles = neoplastic cells anchoring to stromal vessels, shorter processes than in ependymal pseudorosettes | |||
**[[Astroblastoma]]. | |||
**[[Glioblastoma]]. | |||
*Multilayered rosettes | |||
**[[Ependymomblastoma]]s. | |||
**[[Medulloepithelioma]]s. | |||
====Other==== | *Meningeothelial rosettes | ||
**[[Meningioma]] - a rare pattern.<ref>{{Cite journal | last1 = Liverman | first1 = C. | last2 = Mafra | first2 = M. | last3 = Chuang | first3 = SS. | last4 = Shivane | first4 = A. | last5 = Chakrabarty | first5 = A. | last6 = Highley | first6 = R. | last7 = Hilton | first7 = DA. | last8 = Byrne | first8 = NP. | last9 = Wesseling | first9 = P. | title = A clinicopathologic study of 11 rosette-forming meningiomas: a rare and potentially confusing pattern. | journal = Acta Neuropathol | volume = 130 | issue = 2 | pages = 311-3 | month = Aug | year = 2015 | doi = 10.1007/s00401-015-1456-6 | PMID = 26106026 }}</ref> | |||
<gallery> | |||
Image:Neuroblastoma Homer Wright rosettes HE.jpg | Homer-Wright rosettes (WC). | |||
Image:RGNT HE 2.jpg | Neurocytic rosettes (WC). | |||
Image:Ependymoma H&E.jpg | Perivascular pseudorosette (WC). | |||
Image:Ependymoblastoma ETMRjpg.jpg | Multilayered rosettes (WC). | |||
Image:Ependymoblastomatous Rosette.jpg | Ependymoblastous rosettes (WC/Marvin101). | |||
Image:Ependymoma true ependymal rosettes and pseudorosettes.jpg | True ependymal rosettes and pseudorosettes (WC). | |||
Image:Astroblastoma HE Specimen.jpg | Radial (cartwheel) profiles (WC). | |||
</gallery> | |||
====Other important histological features==== | |||
*Rosenthal fibres = worm-like or corkscrew-like (brightly) eosinophilic bodies; 10-40 micrometers. | *Rosenthal fibres = worm-like or corkscrew-like (brightly) eosinophilic bodies; 10-40 micrometers. | ||
**Key feature: variable thickness; helps separate from RBCs. | **Key feature: variable thickness; helps separate from RBCs. | ||
| Line 219: | Line 368: | ||
Image:Rosenthal_fibers.jpg | Rosenthal fibres - smear (WC/AFIP) | Image:Rosenthal_fibers.jpg | Rosenthal fibres - smear (WC/AFIP) | ||
</gallery> | </gallery> | ||
*Eosinophilic granular bodies = related to Rosenthal fibres; round cytoplasmic hyaline droplets in | *Eosinophilic granular bodies = related to Rosenthal fibres; round cytoplasmic hyaline droplets in [[astrocyte]]s.<ref>{{Ref MBNP|11}}</ref> | ||
<gallery> | |||
File:Pilocytic Micro.jpg | Eosinophilic granular bodies in pilocytic astrocytoma smear (WC/AFIP) | |||
File:PXA HE x20.jpg | Eosinophilic granular body in a pleomorphic xanthoastrocytoma (WC/jensflorian) | |||
</gallery> | |||
*Pseudopalisading - picket fence-like alignment of cells; long axis of cells perpendicular to interface with other structures/cells. | *Pseudopalisading - picket fence-like alignment of cells; long axis of cells perpendicular to interface with other structures/cells. | ||
**Pseudopalisading of tumour cells (around necrotic regions) is seen in [[glioblastoma]]. | **Pseudopalisading of tumour cells (around necrotic regions) is seen in [[glioblastoma]]. | ||
<gallery> | |||
File:GBM pseudopalisading necrosis.jpg | Pseudopalisading necrosis in a glioblastoma (WC/jensflorian) | |||
</gallery> | |||
*Perivascular lymphocytic cuffing - Lymphocytes surrounding vessels. | |||
** Seen in many inflammatory conditions including MS. | |||
** Often seen in [[ganglioglioma]] and [[pleomorphic xanthoastrocytoma]]. | |||
** Less common in some gemistocytic [[astrocytoma]]. | |||
*** No association with survival. <ref>{{Cite journal | last1 = Rossi | first1 = ML. | last2 = Jones | first2 = NR. | last3 = Candy | first3 = E. | last4 = Nicoll | first4 = JA. | last5 = Compton | first5 = JS. | last6 = Hughes | first6 = JT. | last7 = Esiri | first7 = MM. | last8 = Moss | first8 = TH. | last9 = Cruz-Sanchez | first9 = FF. | title = The mononuclear cell infiltrate compared with survival in high-grade astrocytomas. | journal = Acta Neuropathol | volume = 78 | issue = 2 | pages = 189-93 | month = | year = 1989 | doi = | PMID = 2750489 }}</ref> | |||
<gallery> | |||
File:Ganglioglioma lymphocytic cuffing PAS.jpg | Lymphocytic cuffing in [[ganglioglioma]] (WC/jensflorian) | |||
</gallery> | |||
Note: | Note: | ||
| Line 233: | Line 398: | ||
<gallery> | <gallery> | ||
Image:Rabies_encephalitis_Negri_bodies_PHIL_3377_lores.jpg | Negri bodies. (WC/CDC) | Image:Rabies_encephalitis_Negri_bodies_PHIL_3377_lores.jpg | Negri bodies. (WC/CDC) | ||
</gallery> | |||
*Owl eye inclusions. | |||
**Basiopilic neuronal inclusions in enlarged cells, typically seen in CMV encephalitis | |||
<gallery> | |||
Image:CMV encephalitis owl eye inclusions HE stain.jpg | Owl eye inclusions | |||
</gallery> | </gallery> | ||
| Line 248: | Line 418: | ||
! Image | ! Image | ||
|- | |- | ||
| | | Grumose bodies<br>[[AKA]] granular bodies | ||
| granular and eosinophilic ~50 micrometers | | granular and eosinophilic ~50 micrometers | ||
| neurodegenerative disease, neuroaxonal dystrophies, aging | | neurodegenerative disease, neuroaxonal dystrophies, aging | ||
| Line 336: | Line 506: | ||
Standard work-up: | Standard work-up: | ||
*GFAP. | *GFAP. | ||
* | *MAP2C. <ref>{{Cite journal | last1 = Blümcke | first1 = I. | last2 = Müller | first2 = S. | last3 = Buslei | first3 = R. | last4 = Riederer | first4 = BM. | last5 = Wiestler | first5 = OD. | title = Microtubule-associated protein-2 immunoreactivity: a useful tool in the differential diagnosis of low-grade neuroepithelial tumors. | journal = Acta Neuropathol | volume = 108 | issue = 2 | pages = 89-96 | month = Aug | year = 2004 | doi = 10.1007/s00401-004-0873-8 | PMID = 15146346 }}</ref> | ||
*Ki-67. | *Ki-67 (MIB-1). | ||
Useful additional markers: | |||
*IDH1(R132H) in Astrocytic/Oligodendroglial tumors. <ref>{{Cite journal | last1 = Paulus | first1 = W. | title = GFAP, Ki67 and IDH1: perhaps the golden triad of glioma immunohistochemistry. | journal = Acta Neuropathol | volume = 118 | issue = 5 | pages = 603-4 | month = Nov | year = 2009 | doi = 10.1007/s00401-009-0600-6 | PMID = 19847448 }}</ref> | |||
*ATRX in mixed gliomas. <ref>{{Cite journal | last1 = Reuss | first1 = DE. | last2 = Sahm | first2 = F. | last3 = Schrimpf | first3 = D. | last4 = Wiestler | first4 = B. | last5 = Capper | first5 = D. | last6 = Koelsche | first6 = C. | last7 = Schweizer | first7 = L. | last8 = Korshunov | first8 = A. | last9 = Jones | first9 = DT. | title = ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. | journal = Acta Neuropathol | volume = 129 | issue = 1 | pages = 133-46 | month = Jan | year = 2015 | doi = 10.1007/s00401-014-1370-3 | PMID = 25427834 }}</ref> | |||
*EMA in Ependymal tumors. <ref>{{Cite journal | last1 = Hasselblatt | first1 = M. | last2 = Paulus | first2 = W. | title = Sensitivity and specificity of epithelial membrane antigen staining patterns in ependymomas. | journal = Acta Neuropathol | volume = 106 | issue = 4 | pages = 385-8 | month = Oct | year = 2003 | doi = 10.1007/s00401-003-0752-8 | PMID = 12898159 }}</ref> | |||
*OLIG-2 usually -ve in Ependymomas. <ref>{{Cite journal | last1 = Ishizawa | first1 = K. | last2 = Komori | first2 = T. | last3 = Shimada | first3 = S. | last4 = Hirose | first4 = T. | title = Olig2 and CD99 are useful negative markers for the diagnosis of brain tumors. | journal = Clin Neuropathol | volume = 27 | issue = 3 | pages = 118-28 | month = | year = | doi = | PMID = 18552083 }}</ref><ref>{{Cite journal | last1 = Otero | first1 = JJ. | last2 = Rowitch | first2 = D. | last3 = Vandenberg | first3 = S. | title = OLIG2 is differentially expressed in pediatric astrocytic and in ependymal neoplasms. | journal = J Neurooncol | volume = 104 | issue = 2 | pages = 423-38 | month = Sep | year = 2011 | doi = 10.1007/s11060-010-0509-x | PMID = 21193945 }}</ref> | |||
===Neuronal=== | ===Neuronal=== | ||
*Synaptophysin. | *Synaptophysin. | ||
| Line 352: | Line 526: | ||
=Brain tumours= | =Brain tumours= | ||
{{main|Neuropathology tumours}} | {{main|Neuropathology tumours}} | ||
Tumours are a big part of neuropathology. The most common brain tumour is a metastasis. The most common primary | Tumours are a big part of neuropathology. The most common brain tumour (in adults) is a metastasis. The most common primary tumours originating in the brain (in adults) are [[glioma]]s. More than 50% of these are classified as [[glioblastoma]] which has a horrible prognosis. | ||
=Non-tumour= | =Non-tumour= | ||
==Cerebral hemorrhage== | ==Vascular disorders== | ||
===Cerebral hemorrhage=== | |||
:See: ''[[Intracranial hematoma]]'' for intracranial bleeds | :See: ''[[Intracranial hematoma]]'' for intracranial bleeds | ||
| Line 364: | Line 539: | ||
*[[Intracerebral hematoma]]s. | *[[Intracerebral hematoma]]s. | ||
==Duret hematoma== | ===Duret hematoma=== | ||
*[[AKA]] Duret hemorrhage. | *[[AKA]] Duret hemorrhage. | ||
===General=== | ====General==== | ||
*Bleed in the upper brainstem (midbrain and pons). | *Bleed in the upper brainstem (midbrain and pons). | ||
**Thought to be due to transtentorial herniation secondary to supratentorial mass effect (e.g. supratentorial tumour, [[intracranial hemorrhage]]).<ref name=pmid11819006>{{Cite journal | last1 = Parizel | first1 = PM. | last2 = Makkat | first2 = S. | last3 = Jorens | first3 = PG. | last4 = Ozsarlak | first4 = O. | last5 = Cras | first5 = P. | last6 = Van Goethem | first6 = JW. | last7 = van den Hauwe | first7 = L. | last8 = Verlooy | first8 = J. | last9 = De Schepper | first9 = AM. | title = Brainstem hemorrhage in descending transtentorial herniation (Duret hemorrhage). | journal = Intensive Care Med | volume = 28 | issue = 1 | pages = 85-8 | month = Jan | year = 2002 | doi = 10.1007/s00134-001-1160-y | PMID = 11819006 }}</ref> | **Thought to be due to transtentorial herniation secondary to supratentorial mass effect (e.g. supratentorial tumour, [[intracranial hemorrhage]]).<ref name=pmid11819006>{{Cite journal | last1 = Parizel | first1 = PM. | last2 = Makkat | first2 = S. | last3 = Jorens | first3 = PG. | last4 = Ozsarlak | first4 = O. | last5 = Cras | first5 = P. | last6 = Van Goethem | first6 = JW. | last7 = van den Hauwe | first7 = L. | last8 = Verlooy | first8 = J. | last9 = De Schepper | first9 = AM. | title = Brainstem hemorrhage in descending transtentorial herniation (Duret hemorrhage). | journal = Intensive Care Med | volume = 28 | issue = 1 | pages = 85-8 | month = Jan | year = 2002 | doi = 10.1007/s00134-001-1160-y | PMID = 11819006 }}</ref> | ||
*Often fatal.<ref name=pmid11098635>{{Cite journal | last1 = Fujimoto | first1 = Y. | last2 = Aguiar | first2 = PH. | last3 = Freitas | first3 = AB. | last4 = de Andrade | first4 = AF. | last5 = Marino Júnior | first5 = R. | title = Recovery from Duret hemorrhage: a rare complication after craniotomy--case report. | journal = Neurol Med Chir (Tokyo) | volume = 40 | issue = 10 | pages = 508-10 | month = Oct | year = 2000 | doi = | PMID = 11098635 }}</ref> | *Often fatal.<ref name=pmid11098635>{{Cite journal | last1 = Fujimoto | first1 = Y. | last2 = Aguiar | first2 = PH. | last3 = Freitas | first3 = AB. | last4 = de Andrade | first4 = AF. | last5 = Marino Júnior | first5 = R. | title = Recovery from Duret hemorrhage: a rare complication after craniotomy--case report. | journal = Neurol Med Chir (Tokyo) | volume = 40 | issue = 10 | pages = 508-10 | month = Oct | year = 2000 | doi = | PMID = 11098635 }}</ref> | ||
===Gross=== | ====Gross==== | ||
*Extravasated blood in midbrain and pons - usu. ventral (anterior) and paramedian (adjacent to the midline).<ref name=pmid11819006/> | *Extravasated blood in midbrain and pons - usu. ventral (anterior) and paramedian (adjacent to the midline).<ref name=pmid11819006/> | ||
| Line 376: | Line 551: | ||
*[http://library.med.utah.edu/WebPath/jpeg5/CNS037.jpg Duret hemorrhage (med.utah.edu)].<ref>URL: [http://library.med.utah.edu/WebPath/EXAM/IMGQUIZ/npfrm.html http://library.med.utah.edu/WebPath/EXAM/IMGQUIZ/npfrm.html]. Accessed on: 4 December 2011.</ref> | *[http://library.med.utah.edu/WebPath/jpeg5/CNS037.jpg Duret hemorrhage (med.utah.edu)].<ref>URL: [http://library.med.utah.edu/WebPath/EXAM/IMGQUIZ/npfrm.html http://library.med.utah.edu/WebPath/EXAM/IMGQUIZ/npfrm.html]. Accessed on: 4 December 2011.</ref> | ||
===Microscopic=== | ====Microscopic==== | ||
Features: | Features: | ||
*RBC extravasation. | *RBC extravasation. | ||
*+/-Hemosiderin-laden macrophages. | *+/-Hemosiderin-laden macrophages. | ||
*+/-Ischemic neurons. | *+/-Ischemic neurons. | ||
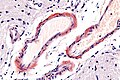
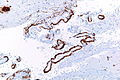
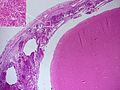
===Cerebral amyloid angiopathy=== | |||
====General==== | |||
*Abbreviated ''CAA''. | |||
*Disease of the old. | |||
*Strong association with ''[[lobar haemorrhage]]'' (bleeds of the cerebellar cortex and cerebral cortex).<ref name=pmid16982664>{{cite journal |author=Thanvi B, Robinson T |title=Sporadic cerebral amyloid angiopathy--an important cause of cerebral haemorrhage in older people |journal=Age Ageing |volume=35 |issue=6 |pages=565–71 |year=2006 |month=November |pmid=16982664 |doi=10.1093/ageing/afl108 |url=}}</ref> | |||
Etiology: | |||
*[[Amyloid]] deposition in the basal lamina of smooth muscle (in the cerebellar cortex and cerebral cortex). | |||
====Gross==== | |||
*Bleeds typically superficial (cortex and subcortical white matter) and in the frontal lobe or parietal lobe.<ref name=pmid17297004>{{Cite journal | last1 = Haacke | first1 = EM. | last2 = DelProposto | first2 = ZS. | last3 = Chaturvedi | first3 = S. | last4 = Sehgal | first4 = V. | last5 = Tenzer | first5 = M. | last6 = Neelavalli | first6 = J. | last7 = Kido | first7 = D. | title = Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. | journal = AJNR Am J Neuroradiol | volume = 28 | issue = 2 | pages = 316-7 | month = Feb | year = 2007 | doi = | PMID = 17297004 | URL = http://www.ajnr.org/content/28/2/316.long }}</ref> | |||
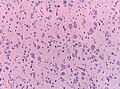
====Microscopic==== | |||
Features: | |||
*Amorphous, acellular eosinophilic material within walls of small arteries. | |||
**This is a high power diagnosis with congo red staining. | |||
Notes: | |||
*Amyloidosis is seen in all individuals with [[Alzheimer's disease]]; the amount of amyloid is what differs -- in CAA it is lots and lots. | |||
*The white matter is typically spared by CAA.<ref name=pmid19225408>{{Cite journal | last1 = Schröder | first1 = R. | last2 = Deckert | first2 = M. | last3 = Linke | first3 = RP. | title = Novel isolated cerebral ALlambda amyloid angiopathy with widespread subcortical distribution and leukoencephalopathy due to atypical monoclonal plasma cell proliferation, and terminal systemic gammopathy. | journal = J Neuropathol Exp Neurol | volume = 68 | issue = 3 | pages = 286-99 | month = Mar | year = 2009 | doi = 10.1097/NEN.0b013e31819a87f9 | PMID = 19225408 }} | |||
</ref> | |||
====Images==== | |||
<gallery> | |||
Image:Cerebral_amyloid_angiopathy_-_very_high_mag.jpg |CAA - congo red - very high mag. (WC) | |||
Image:Cerebral_amyloid_angiopathy_-_low_mag.jpg |CAA - congo red - low mag. (WC) | |||
Image:Cerebral_amyloid_angiopathy_-2b-_amyloid_beta_-_high_mag.jpg |CAA - beta-amyloid - high mag. (WC) | |||
</gallery> | |||
====Stains==== | |||
*[[Congo red]]. | |||
====IHC==== | |||
*Abeta-amyloid (AKA beta-amyloid). | |||
===Cerebral amyloid angiopathy associated with inflammation (I-CAA)=== | |||
* Cognitive decline. | |||
* Microbleedings in MRI. | |||
* Responsive to steroids. | |||
* Abeta deposits in vessels. | |||
* Perivascular lymphocytic infiltrate (but no vasculitis!). | |||
* Giant cells may be present. | |||
===Vascular malformations=== | |||
{{Main|Vascular malformations}} | |||
Types:<ref name=pmid17076525>{{cite journal |author=Prayson RA, Kleinschmidt-DeMasters BK |title=An algorithmic approach to the brain biopsy--part II |journal=Arch. Pathol. Lab. Med. |volume=130 |issue=11 |pages=1639–48 |year=2006 |month=November |pmid=17076525 |doi= |url=}}</ref> | |||
*[[Vascular_malformations#Arteriovenous_malformation|Arteriovenous malformation]]. | |||
*Varix. | |||
*Venous angioma. | |||
*[[Vascular_malformations#Cavernous_hemangioma|Cavernous hemangioma]] (Cavernoma). | |||
*Capillary teleangiectasia. | |||
Also see: ''[[Sturge-Weber syndrome]]''. | |||
===Atherosclerosis=== | |||
{{Main|Vascular_disease#Atherosclerosis}} | |||
*Intracranial atherosclerosis most common at circle of Willis. | |||
*Macroscopic yellow discoloration. | |||
*Luminal stenosis and eccentric intimal thickening. | |||
<gallery> | |||
File:Carotid Plaque (121061911).jpg|Plaque of the carotid bifurcation (Ed Uthman). | |||
</gallery> | |||
===Other large arterial diseases=== | |||
*[[Vascular_disease#Fibromuscular_dysplasia|Fibromuscular dysplasia]]. | |||
*Moyamoya disease. | |||
**Progressive stenosis of basal intracranial arteries and abnormal vascularization. | |||
*[[Aortic dissection|Arterial dissection]]. | |||
*[[Giant cell arteritis]]. | |||
*[[Takayasu's arteritis]]. | |||
*[[Antiphospholipid antibody syndrome|Antiphospholipid antibody]] mediated thrombosis. | |||
===Microangiopathy=== | |||
*Defined as Small vessel disease (<300µm in transverse section). | |||
*Includes atherosclerosis and cerebral amyloid angiopathy. | |||
Other causes: | |||
*Primary angitis of the CNS (PACNS). | |||
*[[Polyarteritis nodosa]]. | |||
*[[Granulomatosis with polyangiitis]] (Wegener's granulomatosis). | |||
*[[Lymphomatoid granulomatosis]]. | |||
*Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (commonly abbreviated ''CADASIL''). | |||
{{Main|Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy}} | |||
===Hypoxic-ischemic encephalopathy=== | |||
*Abbreviated ''HIE''. | |||
{{Main|Hypoxic-ischemic encephalopathy}} | |||
**Hypoxia: reduction in oxygen supply or utilization. | |||
**Ischemia: reduction in blood supply. | |||
===Cerebrovascular accident=== | |||
*Abbreviated ''CVA''. | |||
*[[AKA]] ''stroke''. | |||
{{Main|Cerebrovascular accident}} | |||
*Stroke includes: | |||
**Infarction (ischemia in defined vascular distribution persisting for at least 24hrs). | |||
**Intracrebral hemorrhage (focal blood accumulation in the brain parenchyma). | |||
**Subarachnoid hemorrhage (SAH). | |||
**Cerebral venous thrombosis (CVT). | |||
==Alcohol & CNS== | ==Alcohol & CNS== | ||
| Line 440: | Line 713: | ||
! Protein | ! Protein | ||
! Cells | ! Cells | ||
! Cytopathology | |||
|- | |- | ||
| Bacterial, acute | | Bacterial, acute | ||
| Line 445: | Line 719: | ||
| high | | high | ||
| neutrophils | | neutrophils | ||
| [[File:Purulent_CSF.jpg|100px|center|]][[CSF_cytopathology#Acute_bacterial_meningitis|Cytophathology]] | |||
|- | |- | ||
| Viral | | Viral | ||
| Line 450: | Line 725: | ||
| slight elevation | | slight elevation | ||
| lymphocytes | | lymphocytes | ||
| [[CSF_cytopathology#Viral_meningitis|Cytophathology]] | |||
|} | |} | ||
====Etiology==== | ====Etiology==== | ||
| Line 467: | Line 745: | ||
|- | |- | ||
| Neonate | | Neonate | ||
| ''Escherichia coli'', Group B Streptococcus | | ''Escherichia coli'', ''Group B Streptococcus'' | ||
|- | |- | ||
| Infants, children | | Infants, children | ||
| Line 488: | Line 766: | ||
====Image==== | ====Image==== | ||
<gallery> | <gallery> | ||
Image:Streptococcus_pneumoniae_meningitis,_gross_pathology_33_lores.jpg | Meningitis. (WC) | Image:Streptococcus_pneumoniae_meningitis,_gross_pathology_33_lores.jpg | Streptococcus Meningitis. (WC/Dr. Edwin P. Ewing, Jr.) | ||
File:Haemophilus influenzae meningitis 5003 lores.jpg | Hemophilus influenza Meningitis. (WC/CDC) | |||
File:Pneumococcal meningitis.jpg | Pneumococcus Meningitis. (WC/Dr. Yale Rosen) | |||
File:Meningitis-MRI.JPG | Bacterial Meningitis MRI (WC/MBq) | |||
</gallery> | </gallery> | ||
| Line 506: | Line 787: | ||
====Image==== | ====Image==== | ||
<gallery> | <gallery> | ||
Image:Meningitis_Histopathology.jpg | Bacterial meningitis. (WC) | Image:Meningitis_Histopathology.jpg | Bacterial meningitis. (WC/Marvin101) | ||
</gallery> | </gallery> | ||
| Line 525: | Line 806: | ||
{{Main|Neurodegenerative diseases}} | {{Main|Neurodegenerative diseases}} | ||
This is a hueueuge topic. It is covered in its own article and includes a general discussion of dementia. | This is a hueueuge topic. It is covered in its own article and includes a general discussion of dementia. | ||
==Malformation of cortical development(MCD) == | |||
===Lissencephaly=== | |||
* Greek: ‘lissos': smooth and ‘enkephalos': brain. | |||
* Absent (agyria) or decreased (pachygyria) convolutions. | |||
* Cortical thickening | |||
* Smooth cerebral surface | |||
* Subtypes with different layering: 2-layered, 3-layered, and 4-layered forms. | |||
* Heterotopic neurons in a pattern suggestive of laminar organization. | |||
* 14 LIS mutations account for 90% of all cases.<ref name="pmid27781032">{{cite journal |authors=Parrini E, Conti V, Dobyns WB, Guerrini R |title=Genetic Basis of Brain Malformations |journal=Mol Syndromol |volume=7 |issue=4 |pages=220–233 |date=September 2016 |pmid=27781032 |pmc=5073505 |doi=10.1159/000448639 |url=}}</ref> | |||
===Polymicrogyria=== | |||
* Abnormal cortical lamination. | |||
* Abnormally small and partly fused gyri. | |||
* Can be unilateral, bilateral and symmetrical. | |||
* Intellectual disability. | |||
* Sometimes severe encephalopathy. | |||
* Pharmacoresistant epilepsy | |||
* Cortical lamination can be unlayered or four-layered. | |||
** Unlayered: Unorganized radial distribution of neurons. | |||
** Four-layered: Molecular layer, outer neuronal layer, nerve fiber layer, and inner neuronal layer. | |||
* 1q trisomy in unilateral cases.<ref name="pmid32979071">{{cite journal |authors=Kobow K, Jabari S, Pieper T, Kudernatsch M, Polster T, Woermann FG, Kalbhenn T, Hamer H, Rössler K, Mühlebner A, Spliet WGM, Feucht M, Hou Y, Stichel D, Korshunov A, Sahm F, Coras R, Blümcke I, von Deimling A |title=Mosaic trisomy of chromosome 1q in human brain tissue associates with unilateral polymicrogyria, very early-onset focal epilepsy, and severe developmental delay |journal=Acta Neuropathol |volume=140 |issue=6 |pages=881–891 |date=December 2020 |pmid=32979071 |doi=10.1007/s00401-020-02228-5 |url=}}</ref> | |||
==Epilepsy== | ==Epilepsy== | ||
{{Main|Epilepsy}} | {{Main|Epilepsy}} | ||
===Focal cortical dysplasia (FCD)=== | |||
*Localized malformations of the cortex. | |||
*Frequently associated with epilepsy in children. | |||
*Includes cortical dyslamination, cytoarchitectural changes and white matter abnormalities. | |||
*Current consensus: ILAE classification scheme 2011 <ref>{{Cite journal | last1 = Blümcke | first1 = I. | last2 = Aronica | first2 = E. | last3 = Miyata | first3 = H. | last4 = Sarnat | first4 = HB. | last5 = Thom | first5 = M. | last6 = Roessler | first6 = K. | last7 = Rydenhag | first7 = B. | last8 = Jehi | first8 = L. | last9 = Krsek | first9 = P. | title = International recommendation for a comprehensive neuropathologic workup of epilepsy surgery brain tissue: A consensus Task Force report from the ILAE Commission on Diagnostic Methods. | journal = Epilepsia | volume = 57 | issue = 3 | pages = 348-58 | month = Mar | year = 2016 | doi = 10.1111/epi.13319 | PMID = 26839983 }} | |||
</ref>(based on previous classification by Palmini 2004): | |||
*Type I FCD (focal) | |||
* | **Ia: Abnormal radial cortical lamination. | ||
**Ib: Abnormal tangential cortical lamination. | |||
**Ic: Abnormal radial and tangential cortical lamination. | |||
*Type II FCD (focal) | |||
**IIa: Presence of dysmorphic neurons. | |||
* | **IIb: Presence of dysmorphic neurons and balloon cells. | ||
* | |||
* | |||
*Type III FCD (associated with other lesion) | |||
* | **IIIa: FCD associated with [[Epilepsy#Hippocampal_sclerosis|hippocampal sclerosis]]. | ||
**IIIb: FCD adjacent to a brain tumor. | |||
**IIIc: FCD adjacent to vascular malformation. | |||
**IIIc: FCD associated with previous injury (trauma, inflammation...). | |||
<gallery> | <gallery> | ||
File:FCDIIa dysmorphic neurons HE.jpg|Dysmorphic neurons in FCD (HE) | |||
File:FCDIIa neuronal heterotopia neun.jpg|Heterotopic neurons (NeuN) | |||
</gallery> | </gallery> | ||
=== | ===Hamartia=== | ||
* | * Small collection of ectopic glioneuronal cells. | ||
**Morpholology resembling oligodendroglial-like cells. <ref>{{Cite journal | last1 = Kasper | first1 = BS. | last2 = Stefan | first2 = H. | last3 = Buchfelder | first3 = M. | last4 = Paulus | first4 = W. | title = Temporal lobe microdysgenesis in epilepsy versus control brains. | journal = J Neuropathol Exp Neurol | volume = 58 | issue = 1 | pages = 22-8 | month = Jan | year = 1999 | doi = | PMID = 10068310 }}</ref> | |||
* Mostly amygdala, less common in hippocampus or temporal lobe. | |||
* Can coexist with focal cortical dysplasia. | |||
==Demyelination== | |||
===Multiple sclerosis=== | |||
*Abbreviated ''MS''. | |||
{{Main|Multiple sclerosis}} | |||
===Osmotic demyelination syndrome=== | |||
{{Main|Osmotic demyelination syndrome}} | |||
*Previously known as ''central pontine myelinolysis'' (abbreviated ''CPM''). | |||
===Acute disseminated encephalomyelitis=== | |||
*Abbreviated ''ADEM''. | |||
{{Main|Acute disseminated encephalomyelitis}} | |||
===Neuromyelitis optica=== | |||
*Abbreviated ''NMO''. | |||
General: | |||
*Rare autoimmune disease - once considered a variant of [[multiple sclerosis]]. | |||
*[[ | **Autoantibodies directed at aquaporin-4.<ref name=pmid22087205>{{Cite journal | last1 = Kim | first1 = W. | last2 = Kim | first2 = SH. | last3 = Kim | first3 = HJ. | title = New insights into neuromyelitis optica. | journal = J Clin Neurol | volume = 7 | issue = 3 | pages = 115-27 | month = Sep | year = 2011 | doi = 10.3988/jcn.2011.7.3.115 | PMID = 22087205 }}</ref> | ||
=== | |||
Diagnosis: | |||
* | *NMO-IgG. | ||
Clinical: | Clinical - preferentially: | ||
*Eye (optic neuritis). | |||
* | *Spinal cord (myelitis). | ||
* | |||
Microscopic: | |||
*Inflammation - lymphocytes, macrophages. | |||
* | *Reactive astrocytes. | ||
* | |||
Images: | Images: | ||
*[http:// | *[http://path.upmc.edu/cases/case637.html Neuromyelitis optica - several images (upmc.edu)]. | ||
* | |||
IHC: | |||
*Mixed lymphocyte population with CD3 > CD20. | |||
*Aquaporin-4 loss. | |||
===Progressive multifocal leukoencephalopathy=== | |||
*Abbreviated ''PML''. | |||
{{Main|Progressive multifocal leukoencephalopathy}} | |||
=Cysts= | =Cysts= | ||
| Line 639: | Line 934: | ||
***Usually seen with vertebral anomalies. | ***Usually seen with vertebral anomalies. | ||
*Epithelial cyst. | *Epithelial cyst. | ||
*Cyst with a mural nodule tumor of the brain. | |||
**Commonly seen in: <ref>{{Cite journal | last1 = Raz | first1 = E. | last2 = Zagzag | first2 = D. | last3 = Saba | first3 = L. | last4 = Mannelli | first4 = L. | last5 = Di Paolo | first5 = PL. | last6 = D'Ambrosio | first6 = F. | last7 = Knopp | first7 = E. | title = Cyst with a mural nodule tumor of the brain. | journal = Cancer Imaging | volume = 12 | issue = | pages = 237-44 | month = Aug | year = 2012 | doi = 10.1102/1470-7330.2012.0028 | PMID = 22935908 }}</ref> | |||
**[[Hemangioblastoma]] | |||
**[[Craniopharyngioma]] | |||
**[[Ganglioglioma]] | |||
*Others. | *Others. | ||
| Line 695: | Line 995: | ||
===Epidemiology=== | ===Epidemiology=== | ||
*Autosomal recessive - mutation in a number of genes including NPHP1, AHI1, and CEP290.<ref name=ninds_joubert/> | *Autosomal recessive - mutation in a number of genes including NPHP1, AHI1, and CEP290.<ref name=ninds_joubert/> | ||
=See also= | =See also= | ||
Latest revision as of 17:17, 25 March 2021
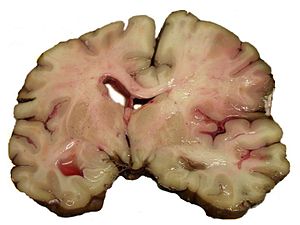
This article is an introduction to neuropathology. There are separate articles for brain tumours, the pituitary gland, the spine, the eye, muscle pathologies, neurohistology and neuroanatomy.
Neuropathology is the bane of many anatomical pathologists in teaching hospitals... 'cause they have to fill in for the neuropathologist when he or she is on vacation.
Neuroanatomy
This is a large topic. It covered in a separate article, that also covers grossing.
Neuroradiology
Key factors to consider in evaluation:
- Location.
- Number of lesions - single versus multiple.
- Cystic versus solid lesion.
- Enhancement.
Lesion location
In neuroradiology and neuropathology, real estate is crucial. Lesion location can often narrow your differential.
Cortical lesions (gray matter):
- Oligodendroglioma.
- DNET.
- Ganglioglioma.
- Pleomorphic xanthoastrocytoma.
- Extraventricular ependymoma.
Cortical-subcortical junction:
- Metastases.
- Abscesses (hematogenous spread).
Subcortical lesions (white matter):
- Glioblastoma.
- Diffuse gliomas.
- Demyelinating plaques.
Deep gray matter lesions (e.g. basal ganglia):
- Gliomas.
- Hypertensive hemorrhage
Cerebellar lesions:
Intraventricular lesions:
- Ependymoma.
- Subependymoma.
- Pilocytic astrocytoma.
- Central neurocytoma.
- Rosette forming glioneuronal tumour of the fourth ventricle.
Suprasellar (above the pituitary):
Number of lesions
If single lesion = think primary, neoplastic If multiple lesions = think metastatic, neoplastic or infectious NB: glioblastoma can be multifocal (and the foci can be quite far apart)
Cystic vs. solid lesions
Some tumours are classically cystic with a small solid component (so-called cyst with a mural nodule) -- e.g. pilocytic astrocytoma, ganglioglioma, hemangioblastoma
Enhancing vs. non-enhancing:
- In adults, enhancing generally = high grade.
- In pediatrics, it often depends on the pattern.
Two main patterns to be mindful of -- ring enhancing lesions, and cystic lesions with a mural nodule.
Ring enhancing lesions
In HIV/AIDS patients... mass on CT if infection:
- Toxoplasmosis - most common.[1]
Ring enhancing lesion (DDx) - mnemonic MAGICAL DR:[2]
- Metstasis.
- Abscess.
- Glioblastoma.
- Infarct.
- Contusion.
- AIDS-related.
- Lymphoma + HIV assoc. disease (toxoplasma).
- Demyelination (e.g. multiple sclerosis).
- Resolving hematoma.
Cyst with enhancing mural nodule
- hemangioblastoma (#1 in adults)
- pilocytic astrocytoma (#1 in peds)
- pleomorphic xanthoastrocytoma
- ganglioglioma
Grossing
This is covered in the neuroanatomy article.
Gross pathology
The gross usually useless for arriving at a definitive diagnosis.
Exceptions:[3]
- Sausage shape lesion of filum terminale = myxopapillary ependymoma.
- Soft & tan colour = pituitary adenoma.
Normal histology
This is a big topic. It is covered in a separate article called neurohistology.
Histopathology
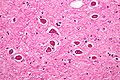
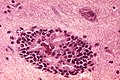
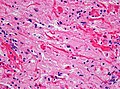
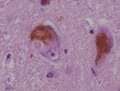
Neuronal changes
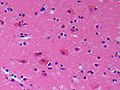
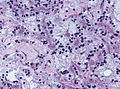
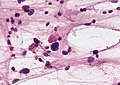
Anoxic neurons
- AKA red neurons.
Features:
- Intensely red cytoplasm.
- Pyknosis = nuclear shrinkage + darker staining.
Images
www:
Central chromatolysis
Features:[6]
- Central clearing.
- Nucleus and Nissl substance are pushed to cell periphery.
DDx:
Images
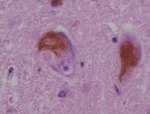
Axonal swellings
H&E:
- Eosinophilic (light pink) - ground glass-like appearance.
- Shape:
- Round if sectioned perpendicular to axis of axon.
- Bound by cell membrane.
- Large ~ typically 2-4x RBC diameter.
- Sausage-shaped if cut in along axis.
- Round if sectioned perpendicular to axis of axon.
Images:
IHC
- APP.
Image:
Glial changes
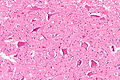
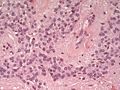
Astrocyte changes
Reactive astrocytes:
- Approximately equally-spaced; distance between neighbouring astrocytes is ~2x (or more) the cell size.
- Well-defined cell border.
- Eosinophilic cytoplasm with many branching processes.
- Classically described as "funnel-shaped" in benign astrocytes.[11]
- Peripheral nucleus.
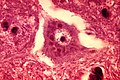
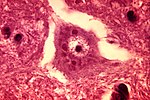
Alzheimer type II astrocyte:[12]
- Large cleared nucleus - key feature.
- Indistinct cytoplasm.
- Found in the context of hepatic encephalopathy in basal ganglia and lower layers of cortex.[13]
- Images:
Creutzfeldt cell:[14]
- Astrocyte that mimics a mitoses; has moderate (identifiable) cytoplasm.
- Finding associated with demyelinating disease.
- Image: Crutzfeldt cell (upmc.edu).[15]
Gemistocytic astrocytes:[16]
- Distinct eosinophilic cytoplasm - with ground-glass appearance.
Tufted astrocytes:[17]
- Cellular processes loaded with tau protein (as may be seen with tau IHC or Gallyas silver stain); Parisian-star-like appearance with special stain.
- +/-Multinucleated.
- A classic feature of progressive supranuclear palsy.
Other glial
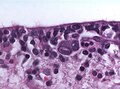
Bergmann gliosis (in the cerebellum):[14]
- Thin layer of cells (2-3 cells) with open nuclei that are larger than granular cell layer nuclei; seen with Purkinje cell loss.
Image:
Reactive change vs. malignancy
Reactive changes vs. malignancy (mnemonic MIMICS):[18]
- MIcrovesicular pattern.
- Mitoses.
- Irregular spacing.
- Calcifications.
- Satellitosis, perineuronal.
- Large "crowds" of glial cells associated with nuclei.
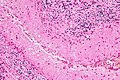
Inflammatory
DDx:
- Autoimmune - Multiple sclerosis.
- Neoplastic - CNS lymphoma.
- Infectious - HSV.
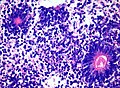
Encephalitis
see also:
General
DDx:
- Viral encephalitis (Neurotrophic viruses):[19]
- Eteroviruses are the most common cause of aseptic meningitis.
- Coxackie Virus.
- Enteric cytopathic human orphan (ECHO) virus.
- Human Herpesviruses (HSV1, HSV2, VZV, CMV, EBV, Roseola)
- HSV encephalitis has high mortality without acyclovir treatment.
- Childhood cerebellitis mainly associated with varicella.
- VZV is the second most common viral meningitis after enterovirus.
- Measles virus(worldwide more than 100.000 deaths annually).
- Is linked to acute demyelinating encephalomyelitis (ADEM) and Subacute sclerosing encephalitis (SSPE).
- Seasonal influenza A virus (highest patogenic potential: avian influenza H5N1).
- Polio and Non-Polio Enterovirus (mostly children).
- Although massive eradication: Polio still existent in Pakistan, Afghanistan, Nigeria.
- Rabies virus
- Tick-borne encephalitis virus (Europe, Siberia, Russian far-east).
- West-Nile virus (US, Southern europe).
- St. Louis encephalitis virus (US).
- Japanese encephalitis virus (South, south-east asia, high disability rate).
- La Crosse virus (esp. children, midwest & eastern US).
- Borna disease virus (VSBV-1).
- Equine encephalitis viruses (EEEV, VEEV, WEEV, CHIKV).
- Eteroviruses are the most common cause of aseptic meningitis.
- Paraneoplastic syndromes.
- Autoimmune antibody-mediated limbic encephalitis (NMDAR).
- Purulent bacterial encephalitis
- Streptococcus, Actinomyces ....
- Septic metastatic encephalitis
- microabscesses, local astrogliosis, two or more granulocytic infiltrates without relation to vessel.[20]
- Septic embolic encephalitis
- Embolic endocarditis, Stroke-like lesions.[21]
- Non-purulent bacterial encephalitis
- Tuberculosis...
Gross
- Frontal and temporal lobe - most common for HSV encephalitis.[22]
Microscopic
Features:[23]
- Perivascular inflammation.
- Microglia.
- +/-Neuronophagia.
- Phagocytosis of neurons.[24]
- +/-Viral cytopathic changes.
- +/-Perineuronal inflammation.
Notes:
- Hemorrhage[25] and necrosis - characteristic of HSV encephalitis.
Image:
IHC
IHC stains for:
- Viral etiologies, e.g. HSV, CMV.
- Parasites, e.g. toxoplasma.
- Fungi, e.g. PASD.
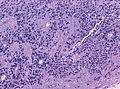
Vasculitis
DDx Cerebral vasculitis / angiitis:
- Systemic lupus erythematosus (SLE)
- Rheumatoid arthritis.
- Medications and drugs (amphetamine, cocaine and heroin).
- Paraneoplastic(lymphomas, leukemia and lung cancer).
- Granulomatosis_with_polyangiitis
- Giant cell arteritis
- Takayasu's arteritis
- Polyarteritis nodosa
- Beta-amyloid-related angiitis (ABRA)
Architecture
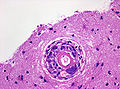
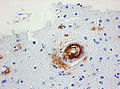
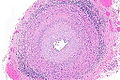
Rosettes
- Rosette = circular/flower-like arrangement of cells.[27]
- Perivascular pseudorosette = circular/flower-like arrangement of cells with blood vessel at the centre.[27]
- Homer-Wright rosette = (circular) rosette with a small (~100 micrometers ???) meshwork of fibers (neuropil) at the centre.[27]
- Medulloblastoma.
- Image: Medulloblastoma (ouhsc.edu).[28]
- PNET (can be thought of as a supratentorial medulloblastoma) .
- Neuroblastoma
- Medulloblastoma.
- Flexner-Wintersteiner rosette = rosette with empty centre (donut hole).[27]
- True ependymal rosette = surrounds a space.[27]
- Pineocytomatous/neurocytic rosette = irregular rosette with a large meshwork of fibers (neuropil) at the centre.[27]
- Similar to Homer-Wright rosette.
- Pineocytoma.
- Neurocytoma.
- RGNT - Rosette forming glioneuronal tumor of the IVth ventricle.
- Radial (cartwheel) profiles = neoplastic cells anchoring to stromal vessels, shorter processes than in ependymal pseudorosettes
- Multilayered rosettes
- Meningeothelial rosettes
- Meningioma - a rare pattern.[29]
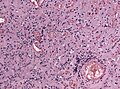
Other important histological features
- Rosenthal fibres = worm-like or corkscrew-like (brightly) eosinophilic bodies; 10-40 micrometers.
- Key feature: variable thickness; helps separate from RBCs.
- Well-seen on trichrome stains.
- Eosinophilic granular bodies = related to Rosenthal fibres; round cytoplasmic hyaline droplets in astrocytes.[30]
- Pseudopalisading - picket fence-like alignment of cells; long axis of cells perpendicular to interface with other structures/cells.
- Pseudopalisading of tumour cells (around necrotic regions) is seen in glioblastoma.
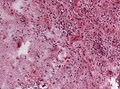
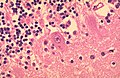
- Perivascular lymphocytic cuffing - Lymphocytes surrounding vessels.
- Seen in many inflammatory conditions including MS.
- Often seen in ganglioglioma and pleomorphic xanthoastrocytoma.
- Less common in some gemistocytic astrocytoma.
- No association with survival. [31]
Lymphocytic cuffing in ganglioglioma (WC/jensflorian)
Note:
- Good set of articles: Neuropathology for radiologists (ncbi.nlm.nih.gov).
Inclusion bodies
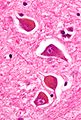
- Negri bodies.
- Cytoplasmic inclusions; classically in Purkinje cells of the cerebellum, pyramidal cells of Ammon's horn.
- Rabies.
- Owl eye inclusions.
- Basiopilic neuronal inclusions in enlarged cells, typically seen in CMV encephalitis
- Lewy bodies.
- Eosinophilic cytoplasmic inclusion - composed mostly of alpha-synuclein.[32]
Table of inclusions
| Feature | Appearance | Associated disease | Comment | Image |
|---|---|---|---|---|
| Grumose bodies AKA granular bodies |
granular and eosinophilic ~50 micrometers | neurodegenerative disease, neuroaxonal dystrophies, aging | ?Comment | ?Image |
| Cowdry type 1 AKA Cowdry type A |
eosinophilic & round + halo | herpes simplex virus | can be confused with Lewy body, Marinesco body |
?Image |
| Lewy body | round cytoplasmic eosinophilic body +/- pale halo |
Parkinson disease, dementia with Lewy bodies | morphology dependent on location in brain; +ve for alpha-synuclein, alpha-B crystallin, ubiquitin |
, [1] |
| Lafora body | round | myoclonic epilepsy | look like corpora amylacea; location: dentate nucleus, liver, skeletal muscle, sweat glands | ?Image |
| Lipofuscin | yellow & granular | aging | olive, dendate | ?Image |
| Negri body | small eosinophic bodies | rabies | found in hippocampal neurons and Purkinje cells | |
| Hirano body | concentric calcification/rod-shaped bright eosinophilic; overlap edge of neuron | Alzheimer disease, Pick disease[33] | actin crystals, may look like capillaries; location: CA1 of hippocampus | [2][34] |
| Neurofibrillary tangles | flame-shaped cytoplasmic thingy ~30 micrometers |
aging, Alzheimer's disease | seen with silver stain | Schematic[34], [3][35] |
| Granulovacuolar degeneration | cytoplasmic vacuoles 4-5 micrometers | ageing, Alzheimer's disease, Pick's disease |
main found in Ammon horn[33] | [4][35] |
| Pick bodies | round, homogenous, intracytoplasmic, ~10 micrometers | Pick's disease | pyramidal neurons, dentate granule cells (hippocampus); +ve for tau, tubulin, ubiquitin |
[5] |
| Bunina body | size of Nissl granules, eosinophilic | amyotrophic lateral sclerosis (ALS) | EM: membrane-bound bodies; ubiquitin +ve | [6] |
Image collection: Inclusion bodies (photobucket.com).
Immunohistochemistry
General
- S-100.
- Sensitive... but non-specific, e.g. also stains melanoma.
Glial
- GFAP (glial fibrillary acidic protein) - should stain perikaryon.
Glial tumours
Standard work-up:
- GFAP.
- MAP2C. [36]
- Ki-67 (MIB-1).
Useful additional markers:
- IDH1(R132H) in Astrocytic/Oligodendroglial tumors. [37]
- ATRX in mixed gliomas. [38]
- EMA in Ependymal tumors. [39]
- OLIG-2 usually -ve in Ependymomas. [40][41]
Neuronal
- Synaptophysin.
- Chromogranin.
Carcinoma vs. glial tumours
- AE1/AE3 often +ve in glial tumours (e.g. astrocytomas, oligodendrogliomas); CAM5.2 is usu. -ve in glial tumours.[42]
Others
- APP (amyloid precursor protein) - detects axonal swellings.
- NF (neurofilament) - detects axonal swellings.
Brain tumours
Tumours are a big part of neuropathology. The most common brain tumour (in adults) is a metastasis. The most common primary tumours originating in the brain (in adults) are gliomas. More than 50% of these are classified as glioblastoma which has a horrible prognosis.
Non-tumour
Vascular disorders
Cerebral hemorrhage
- See: Intracranial hematoma for intracranial bleeds
Includes discussion of:
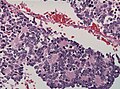
Duret hematoma
- AKA Duret hemorrhage.
General
- Bleed in the upper brainstem (midbrain and pons).
- Thought to be due to transtentorial herniation secondary to supratentorial mass effect (e.g. supratentorial tumour, intracranial hemorrhage).[43]
- Often fatal.[44]
Gross
- Extravasated blood in midbrain and pons - usu. ventral (anterior) and paramedian (adjacent to the midline).[43]
Image:
Microscopic
Features:
- RBC extravasation.
- +/-Hemosiderin-laden macrophages.
- +/-Ischemic neurons.
Cerebral amyloid angiopathy
General
- Abbreviated CAA.
- Disease of the old.
- Strong association with lobar haemorrhage (bleeds of the cerebellar cortex and cerebral cortex).[46]
Etiology:
- Amyloid deposition in the basal lamina of smooth muscle (in the cerebellar cortex and cerebral cortex).
Gross
- Bleeds typically superficial (cortex and subcortical white matter) and in the frontal lobe or parietal lobe.[47]
Microscopic
Features:
- Amorphous, acellular eosinophilic material within walls of small arteries.
- This is a high power diagnosis with congo red staining.
Notes:
- Amyloidosis is seen in all individuals with Alzheimer's disease; the amount of amyloid is what differs -- in CAA it is lots and lots.
- The white matter is typically spared by CAA.[48]
Images
Stains
IHC
- Abeta-amyloid (AKA beta-amyloid).
Cerebral amyloid angiopathy associated with inflammation (I-CAA)
- Cognitive decline.
- Microbleedings in MRI.
- Responsive to steroids.
- Abeta deposits in vessels.
- Perivascular lymphocytic infiltrate (but no vasculitis!).
- Giant cells may be present.
Vascular malformations
Types:[49]
- Arteriovenous malformation.
- Varix.
- Venous angioma.
- Cavernous hemangioma (Cavernoma).
- Capillary teleangiectasia.
Also see: Sturge-Weber syndrome.
Atherosclerosis
- Intracranial atherosclerosis most common at circle of Willis.
- Macroscopic yellow discoloration.
- Luminal stenosis and eccentric intimal thickening.
Other large arterial diseases
- Fibromuscular dysplasia.
- Moyamoya disease.
- Progressive stenosis of basal intracranial arteries and abnormal vascularization.
- Arterial dissection.
- Giant cell arteritis.
- Takayasu's arteritis.
- Antiphospholipid antibody mediated thrombosis.
Microangiopathy
- Defined as Small vessel disease (<300µm in transverse section).
- Includes atherosclerosis and cerebral amyloid angiopathy.
Other causes:
- Primary angitis of the CNS (PACNS).
- Polyarteritis nodosa.
- Granulomatosis with polyangiitis (Wegener's granulomatosis).
- Lymphomatoid granulomatosis.
- Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (commonly abbreviated CADASIL).
Hypoxic-ischemic encephalopathy
- Abbreviated HIE.
- Hypoxia: reduction in oxygen supply or utilization.
- Ischemia: reduction in blood supply.
Cerebrovascular accident
- Abbreviated CVA.
- AKA stroke.
- Stroke includes:
- Infarction (ischemia in defined vascular distribution persisting for at least 24hrs).
- Intracrebral hemorrhage (focal blood accumulation in the brain parenchyma).
- Subarachnoid hemorrhage (SAH).
- Cerebral venous thrombosis (CVT).
Alcohol & CNS
Clinical
- Wernicke's encephalopathy
- Mnemonic WACO:
- Wernicke's.
- Ataxia.
- Confusion, confabulation -- Korsakoff.
- Ocular Sx (CN IV palsy).
- Cause: thiamine deficiency.
- Mnemonic WACO:
Pathology
Features:[50]
- Morel's laminar sclerosis = spongy degeneration and gliosis of the cerebral cortex[51] usu. prominent in the third layer of the cortex (outer pyramidal layer) and especially in the lateral-frontal cortex.[52]
- Central pontine myelinolysis (CPM).[53]
- Just what it sound like - myelin loss in the central pons.
- Classically associated with rapid correction of hyponatremia.[54]
- Mammillary body shrinkage.[55]
- Anterior cerebellar vermis atrophy; weak finding - as also age-related.[56]
- Vermis atrophy is also seen in schizophrenia.[57]
Marchiafava-Bignami Disease
- Rare.
- Demyelination of the corpus callosum.[52]
Wernicke's encephalopathy
General:
- Due to thiamine deficiency.
- Malnourishment often accompanies alcoholism.
Features:[58]
- Neurons of mammillary bodies preserved - key.
- Loss of myelin.
- Hemorrhage.
- Edema.
- Reactive blood vessels.
Note:
- The thalamus and inferior olives show neuronal loss.[58]
Common non-specific findings
- Intracranial haemorrhage - due to trauma.
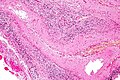
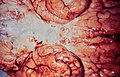
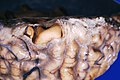
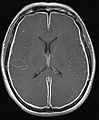
Meningitis
General
- Definition: inflammation of the meninges (pia mater, arachnoid membranes, dura mater).
Classic clinical presentation:
- Neck stiffness.
- Fever.
- +/-Headache.
- +/-Decreased level of consciousness.
CSF findings:
| Type | Glucose | Protein | Cells | Cytopathology |
|---|---|---|---|---|
| Bacterial, acute | low | high | neutrophils | Cytophathology |
| Viral | normal | slight elevation | lymphocytes | Cytophathology |
Etiology
- Infectious.
- Bacterial.
- Viral.
- Parasitic
- Autoimmune.
- Toxic.
- Aseptic - see Mollaret's meningitis.
Bacterial meningitis - most probably cause by age:[59]
| Age | Organism |
|---|---|
| Neonate | Escherichia coli, Group B Streptococcus |
| Infants, children | Streptococcus pneumoniae |
| Adolescents, young adults | Neisseria meningitidis |
| Elderly | Streptococcus pneumoniae, Listeria monocytogenes |
Gross
Features:
- +/-Clouded appearance of the meninges.
- +/-Pus.
- +/-Petechiae.
- +/-Cerebral edema.
Image
Microscopic
Features:
- Inflammation of the meninges:
- +/-Neutrophils.
- +/-Lymphocytes.
- +/-Plasma cells.
- +/-Microorganisms (infectious meningitis):
- Bacteria.
- Fungi, e.g. aspergillosis (may be intravascular).
Main DDx:
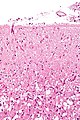
Image
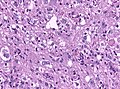
Cerebral abscess
General
- May mimic malignancy clinically.
Microscopic
Features:
- Sheets of neutrophils surrounded by fibrosing brain.
- Fibrosing brain: pale (lighter pink than normal brain tissue), dense.
Images:
Neurodegenerative diseases
This is a hueueuge topic. It is covered in its own article and includes a general discussion of dementia.
Malformation of cortical development(MCD)
Lissencephaly
- Greek: ‘lissos': smooth and ‘enkephalos': brain.
- Absent (agyria) or decreased (pachygyria) convolutions.
- Cortical thickening
- Smooth cerebral surface
- Subtypes with different layering: 2-layered, 3-layered, and 4-layered forms.
- Heterotopic neurons in a pattern suggestive of laminar organization.
- 14 LIS mutations account for 90% of all cases.[61]
Polymicrogyria
- Abnormal cortical lamination.
- Abnormally small and partly fused gyri.
- Can be unilateral, bilateral and symmetrical.
- Intellectual disability.
- Sometimes severe encephalopathy.
- Pharmacoresistant epilepsy
- Cortical lamination can be unlayered or four-layered.
- Unlayered: Unorganized radial distribution of neurons.
- Four-layered: Molecular layer, outer neuronal layer, nerve fiber layer, and inner neuronal layer.
- 1q trisomy in unilateral cases.[62]
Epilepsy
Focal cortical dysplasia (FCD)
- Localized malformations of the cortex.
- Frequently associated with epilepsy in children.
- Includes cortical dyslamination, cytoarchitectural changes and white matter abnormalities.
- Current consensus: ILAE classification scheme 2011 [63](based on previous classification by Palmini 2004):
- Type I FCD (focal)
- Ia: Abnormal radial cortical lamination.
- Ib: Abnormal tangential cortical lamination.
- Ic: Abnormal radial and tangential cortical lamination.
- Type II FCD (focal)
- IIa: Presence of dysmorphic neurons.
- IIb: Presence of dysmorphic neurons and balloon cells.
- Type III FCD (associated with other lesion)
- IIIa: FCD associated with hippocampal sclerosis.
- IIIb: FCD adjacent to a brain tumor.
- IIIc: FCD adjacent to vascular malformation.
- IIIc: FCD associated with previous injury (trauma, inflammation...).
Hamartia
- Small collection of ectopic glioneuronal cells.
- Morpholology resembling oligodendroglial-like cells. [64]
- Mostly amygdala, less common in hippocampus or temporal lobe.
- Can coexist with focal cortical dysplasia.
Demyelination
Multiple sclerosis
- Abbreviated MS.
Osmotic demyelination syndrome
- Previously known as central pontine myelinolysis (abbreviated CPM).
Acute disseminated encephalomyelitis
- Abbreviated ADEM.
Neuromyelitis optica
- Abbreviated NMO.
General:
- Rare autoimmune disease - once considered a variant of multiple sclerosis.
- Autoantibodies directed at aquaporin-4.[65]
Diagnosis:
- NMO-IgG.
Clinical - preferentially:
- Eye (optic neuritis).
- Spinal cord (myelitis).
Microscopic:
- Inflammation - lymphocytes, macrophages.
- Reactive astrocytes.
Images:
IHC:
- Mixed lymphocyte population with CD3 > CD20.
- Aquaporin-4 loss.
Progressive multifocal leukoencephalopathy
- Abbreviated PML.
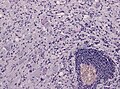
Cysts
General
- All are "benign", but some may be fatal due to spatial constraints.
List of cysts
- Colloid cyst.
- Columnar epithelium.
- Arachnoid cyst - considered precursor of meningioma.
- Psammoma bodies.
- Clumps of cells.
- Whorled pattern.
- Dermoid cyst.
- Skin + adnexal structures.
- ... think of ovarian dermoid.
- Epidermoid cyst.
- Choroid plexus cyst.
- Neuroenteric cyst.
- Foregut cyst with connection to dura.[66]
- Gastrointestinal tract epithelium.
- Usually seen with vertebral anomalies.
- Foregut cyst with connection to dura.[66]
- Epithelial cyst.
- Cyst with a mural nodule tumor of the brain.
- Commonly seen in: [67]
- Hemangioblastoma
- Craniopharyngioma
- Ganglioglioma
- Others.
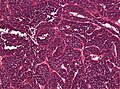
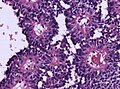
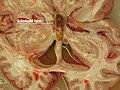
Colloid cyst
General
Classic presentation:[68]
- Headache - may be relieved by lying down.
- Can cause sudden natural death.[69]
Gross
- Fluid filled cyst - classically in the third ventricle.
Images
Colloid cyst at autopsy. (Shaktawat et al.[70])
www:
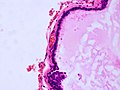
Microscopic
Features:[71]
- Simple epithelium with ciliated cells and goblet cells.
Images
www:
Paediatric pathology
Kernicterus
General
- Due to hyperbilirubinemia.[72]
Gross
Note:
- May not be specific.[72]
Image:
Microscopic
Features - similar to HIE:[73]
- +/-Red neurons.
- +/-Gliosis.
Joubert syndrome
- Malformation of the cerebellar vermis.[76]
Epidemiology
- Autosomal recessive - mutation in a number of genes including NPHP1, AHI1, and CEP290.[76]
See also
References
- ↑ MUN. Feb 3, 2009.
- ↑ Yeung, J.C.; Leonard, Blair J. N. (2005). The Toronto Notes 2005 - Review for the MCCQE and Comprehensive Medical Reference (2005 ed.). The Toronto Notes Inc. for Medical Students Inc.. pp. NS7. ISBN 978-0968592854.
- ↑ R. Kiehl. 8 November 2010.
- ↑ URL: http://neuropathology-web.org/chapter2/chapter2aHIE.html. Accessed on: 10 December 2014.
- ↑ URL: http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/N1-MS-01-16-Ans.htm and http://moon.ouhsc.edu/kfung/iacp-olp/apaq-text/n1-ms-01.htm. Accessed on: 31 October 2010.
- ↑ URL: http://www.neuropathologyweb.org/chapter1/chapter1aNeurons.html. Accessed on: 22 December 2010.
- ↑ Holland GR (1996). "Experimental trigeminal nerve injury". Crit. Rev. Oral Biol. Med. 7 (3): 237–58. PMID 8909880.
- ↑ Piercecchi-Marti MD, Pélissier-Alicot AL, Leonetti G, Tervé JP, Cianfarani F, Pellissier JF (December 2004). "Pellagra: a rare disease observed in a victim of mental and physical abuse". Am J Forensic Med Pathol 25 (4): 342–4. PMID 15577526.
- ↑ URL: http://frontalcortex.com/?page=oll&topic=24&qid=602. Accessed on: 3 November 2010.
- ↑ Finnie JW, Manavis J, Blumbergs PC, Kuchel TR (November 2000). "Axonal and neuronal amyloid precursor protein immunoreactivity in the brains of guinea pigs given tunicamycin". Vet. Pathol. 37 (6): 677–80. PMID 11105962. http://vet.sagepub.com/content/37/6/677.full.
- ↑ MUN. 15 November 2010.
- ↑ URL: http://www.neuropathologyweb.org/chapter1/chapter1bAstrocytes.html. Accessed on: 2 July 2010.
- ↑ Klatt, Edward C. (2006). Robbins and Cotran Atlas of Pathology (1st ed.). Saunders. pp. 202. ISBN 978-1416002741.
- ↑ 14.0 14.1 Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 18. ISBN 978-0443069826.
- ↑ URL: http://path.upmc.edu/cases/case336.html. Accessed on: 15 January 2012.
- ↑ URL: http://www.neuropathologyweb.org/chapter1/chapter1bAstrocytes.html. Accessed on: 7 November 2010.
- ↑ Gray, Francoise; Poirier, Jacques; De Girolami, Umberto (2003). Escourolle and Poirier's Manual of Basic Neuropathology (4th ed.). Churchill Livingstone. pp. 173. ISBN 978-0750674058.
- ↑ Weedman Molavi, Diana (2008). The Practice of Surgical Pathology: A Beginner's Guide to the Diagnostic Process (1st ed.). Springer. pp. 254. ISBN 978-0387744858.
- ↑ Ludlow, M.; Kortekaas, J.; Herden, C.; Hoffmann, B.; Tappe, D.; Trebst, C.; Griffin, DE.; Brindle, HE. et al. (Feb 2016). "Neurotropic virus infections as the cause of immediate and delayed neuropathology.". Acta Neuropathol 131 (2): 159-84. doi:10.1007/s00401-015-1511-3. PMID 26659576.
- ↑ Tauber, SC.; Bunkowski, S.; Brück, W.; Nau, R. (Dec 2011). "Septic metastatic encephalitis: coexistence of brain damage and repair.". Neuropathol Appl Neurobiol 37 (7): 768-76. doi:10.1111/j.1365-2990.2011.01196.x. PMID 21696418.
- ↑ Bitsch, A.; Nau, R.; Hilgers, RA.; Verheggen, R.; Werner, G.; Prange, HW. (Oct 1996). "Focal neurologic deficits in infective endocarditis and other septic diseases.". Acta Neurol Scand 94 (4): 279-86. PMID 8937541.
- ↑ Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 416 Q47. ISBN 978-1416025887.
- ↑ Takei, H.; Wilfong, A.; Malphrus, A.; Yoshor, D.; Hunter, JV.; Armstrong, DL.; Bhattacharjee, MB. (Aug 2010). "Dual pathology in Rasmussen's encephalitis: a study of seven cases and review of the literature.". Neuropathology 30 (4): 381-91. doi:10.1111/j.1440-1789.2009.01079.x. PMID 20051019.
- ↑ URL: http://medical-dictionary.thefreedictionary.com/neuronophagia. Accessed on: 11 April 2012.
- ↑ Vossough, A.; Zimmerman, RA.; Bilaniuk, LT.; Schwartz, EM. (Apr 2008). "Imaging findings of neonatal herpes simplex virus type 2 encephalitis.". Neuroradiology 50 (4): 355-66. doi:10.1007/s00234-007-0349-3. PMID 18246335.
- ↑ URL: http://neuropathology-web.org/chapter5/chapter5dViruses.html. Accessed on: 27 January 2012.
- ↑ 27.0 27.1 27.2 27.3 27.4 27.5 Wippold FJ, Perry A (March 2006). "Neuropathology for the neuroradiologist: rosettes and pseudorosettes". AJNR Am J Neuroradiol 27 (3): 488–92. PMID 16551982.
- ↑ URL: http://moon.ouhsc.edu/kfung/IACP-OLP/APAQ-Text/N1-TU-01.htm#17. Accessed on: 3 December 2010.
- ↑ Liverman, C.; Mafra, M.; Chuang, SS.; Shivane, A.; Chakrabarty, A.; Highley, R.; Hilton, DA.; Byrne, NP. et al. (Aug 2015). "A clinicopathologic study of 11 rosette-forming meningiomas: a rare and potentially confusing pattern.". Acta Neuropathol 130 (2): 311-3. doi:10.1007/s00401-015-1456-6. PMID 26106026.
- ↑ Gray, Francoise; Poirier, Jacques; De Girolami, Umberto (2003). Escourolle and Poirier's Manual of Basic Neuropathology (4th ed.). Churchill Livingstone. pp. 11. ISBN 978-0750674058.
- ↑ Rossi, ML.; Jones, NR.; Candy, E.; Nicoll, JA.; Compton, JS.; Hughes, JT.; Esiri, MM.; Moss, TH. et al. (1989). "The mononuclear cell infiltrate compared with survival in high-grade astrocytomas.". Acta Neuropathol 78 (2): 189-93. PMID 2750489.
- ↑ Marui W, Iseki E, Kato M, Akatsu H, Kosaka K (August 2004). "Pathological entity of dementia with Lewy bodies and its differentiation from Alzheimer's disease". Acta Neuropathol. 108 (2): 121–8. doi:10.1007/s00401-004-0869-4. PMID 15235805.
- ↑ 33.0 33.1 Gray, Francoise; Poirier, Jacques; De Girolami, Umberto (2003). Escourolle and Poirier's Manual of Basic Neuropathology (4th ed.). Churchill Livingstone. pp. 5. ISBN 978-0750674058.
- ↑ 34.0 34.1 URL: http://www.pakmed.net/academic/age/alz/alz030.htm. Accessed on: 12 November 2010.
- ↑ 35.0 35.1 URL: http://faculty.washington.edu/alexbert/MEDEX/Fall/NeuroPath_Obj.htm. Accessed on: 13 November 2010.
- ↑ Blümcke, I.; Müller, S.; Buslei, R.; Riederer, BM.; Wiestler, OD. (Aug 2004). "Microtubule-associated protein-2 immunoreactivity: a useful tool in the differential diagnosis of low-grade neuroepithelial tumors.". Acta Neuropathol 108 (2): 89-96. doi:10.1007/s00401-004-0873-8. PMID 15146346.
- ↑ Paulus, W. (Nov 2009). "GFAP, Ki67 and IDH1: perhaps the golden triad of glioma immunohistochemistry.". Acta Neuropathol 118 (5): 603-4. doi:10.1007/s00401-009-0600-6. PMID 19847448.
- ↑ Reuss, DE.; Sahm, F.; Schrimpf, D.; Wiestler, B.; Capper, D.; Koelsche, C.; Schweizer, L.; Korshunov, A. et al. (Jan 2015). "ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma.". Acta Neuropathol 129 (1): 133-46. doi:10.1007/s00401-014-1370-3. PMID 25427834.
- ↑ Hasselblatt, M.; Paulus, W. (Oct 2003). "Sensitivity and specificity of epithelial membrane antigen staining patterns in ependymomas.". Acta Neuropathol 106 (4): 385-8. doi:10.1007/s00401-003-0752-8. PMID 12898159.
- ↑ Ishizawa, K.; Komori, T.; Shimada, S.; Hirose, T.. "Olig2 and CD99 are useful negative markers for the diagnosis of brain tumors.". Clin Neuropathol 27 (3): 118-28. PMID 18552083.
- ↑ Otero, JJ.; Rowitch, D.; Vandenberg, S. (Sep 2011). "OLIG2 is differentially expressed in pediatric astrocytic and in ependymal neoplasms.". J Neurooncol 104 (2): 423-38. doi:10.1007/s11060-010-0509-x. PMID 21193945.
- ↑ Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 12. ISBN 978-0443069826.
- ↑ 43.0 43.1 Parizel, PM.; Makkat, S.; Jorens, PG.; Ozsarlak, O.; Cras, P.; Van Goethem, JW.; van den Hauwe, L.; Verlooy, J. et al. (Jan 2002). "Brainstem hemorrhage in descending transtentorial herniation (Duret hemorrhage).". Intensive Care Med 28 (1): 85-8. doi:10.1007/s00134-001-1160-y. PMID 11819006.
- ↑ Fujimoto, Y.; Aguiar, PH.; Freitas, AB.; de Andrade, AF.; Marino Júnior, R. (Oct 2000). "Recovery from Duret hemorrhage: a rare complication after craniotomy--case report.". Neurol Med Chir (Tokyo) 40 (10): 508-10. PMID 11098635.
- ↑ URL: http://library.med.utah.edu/WebPath/EXAM/IMGQUIZ/npfrm.html. Accessed on: 4 December 2011.
- ↑ Thanvi B, Robinson T (November 2006). "Sporadic cerebral amyloid angiopathy--an important cause of cerebral haemorrhage in older people". Age Ageing 35 (6): 565–71. doi:10.1093/ageing/afl108. PMID 16982664.
- ↑ Haacke, EM.; DelProposto, ZS.; Chaturvedi, S.; Sehgal, V.; Tenzer, M.; Neelavalli, J.; Kido, D. (Feb 2007). "Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging.". AJNR Am J Neuroradiol 28 (2): 316-7. PMID 17297004.
- ↑ Schröder, R.; Deckert, M.; Linke, RP. (Mar 2009). "Novel isolated cerebral ALlambda amyloid angiopathy with widespread subcortical distribution and leukoencephalopathy due to atypical monoclonal plasma cell proliferation, and terminal systemic gammopathy.". J Neuropathol Exp Neurol 68 (3): 286-99. doi:10.1097/NEN.0b013e31819a87f9. PMID 19225408.
- ↑ Prayson RA, Kleinschmidt-DeMasters BK (November 2006). "An algorithmic approach to the brain biopsy--part II". Arch. Pathol. Lab. Med. 130 (11): 1639–48. PMID 17076525.
- ↑ http://www.journals.elsevierhealth.com/periodicals/ycdip/article/S0968-6053(07)00035-X/abstract
- ↑ URL: http://content.karger.com/ProdukteDB/produkte.asp?Doi=114939. Accessed on: 22 September 2010.
- ↑ 52.0 52.1 Johkura K, Naito M, Naka T (March 2005). "Cortical involvement in Marchiafava-Bignami disease". AJNR Am J Neuroradiol 26 (3): 670–3. PMID 15760886. http://www.ajnr.org/cgi/content/full/26/3/670.
- ↑ Campbell MC (2010). "Hyponatremia and central pontine myelinolysis as a result of beer potomania: a case report". Prim Care Companion J Clin Psychiatry 12 (4). doi:10.4088/PCC.09l00936ecr. PMC 2983455. PMID 21085565. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2983455/.
- ↑ Bernsen HJ, Prick MJ (September 1999). "Improvement of central pontine myelinolysis as demonstrated by repeated magnetic resonance imaging in a patient without evidence of hyponatremia". Acta Neurol Belg 99 (3): 189–93. PMID 10544728.
- ↑ Shear PK, Sullivan EV, Lane B, Pfefferbaum A (November 1996). "Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia". Alcohol. Clin. Exp. Res. 20 (8): 1489-95. PMID 8947329. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0145-6008&date=1996&volume=20&issue=8&spage=1489.
- ↑ Torvik A (1987). "Brain lesions in alcoholics: neuropathological observations". Acta Med. Scand. Suppl. 717: 47–54. PMID 3478969.
- ↑ Sandyk R, Kay SR, Merriam AE (April 1991). "Atrophy of the cerebellar vermis: relevance to the symptoms of schizophrenia". Int. J. Neurosci. 57 (3-4): 205–12. PMID 1938163.
- ↑ 58.0 58.1 Torvik, A.. "Two types of brain lesions in Wernicke's encephalopathy.". Neuropathol Appl Neurobiol 11 (3): 179-90. PMID 3929155.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 666-7. ISBN 978-1416054542.
- ↑ URL: http://pathology.class.kmu.edu.tw/ch01/Slide4.htm. Accessed on: 1 January 2012.
- ↑ Parrini E, Conti V, Dobyns WB, Guerrini R (September 2016). "Genetic Basis of Brain Malformations". Mol Syndromol 7 (4): 220–233. doi:10.1159/000448639. PMC 5073505. PMID 27781032. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5073505/.
- ↑ Kobow K, Jabari S, Pieper T, Kudernatsch M, Polster T, Woermann FG, Kalbhenn T, Hamer H, Rössler K, Mühlebner A, Spliet WGM, Feucht M, Hou Y, Stichel D, Korshunov A, Sahm F, Coras R, Blümcke I, von Deimling A (December 2020). "Mosaic trisomy of chromosome 1q in human brain tissue associates with unilateral polymicrogyria, very early-onset focal epilepsy, and severe developmental delay". Acta Neuropathol 140 (6): 881–891. doi:10.1007/s00401-020-02228-5. PMID 32979071.
- ↑ Blümcke, I.; Aronica, E.; Miyata, H.; Sarnat, HB.; Thom, M.; Roessler, K.; Rydenhag, B.; Jehi, L. et al. (Mar 2016). "International recommendation for a comprehensive neuropathologic workup of epilepsy surgery brain tissue: A consensus Task Force report from the ILAE Commission on Diagnostic Methods.". Epilepsia 57 (3): 348-58. doi:10.1111/epi.13319. PMID 26839983.
- ↑ Kasper, BS.; Stefan, H.; Buchfelder, M.; Paulus, W. (Jan 1999). "Temporal lobe microdysgenesis in epilepsy versus control brains.". J Neuropathol Exp Neurol 58 (1): 22-8. PMID 10068310.
- ↑ Kim, W.; Kim, SH.; Kim, HJ. (Sep 2011). "New insights into neuromyelitis optica.". J Clin Neurol 7 (3): 115-27. doi:10.3988/jcn.2011.7.3.115. PMID 22087205.
- ↑ URL: http://bhj.org/journal/2003_4502_april/neurentericcyst_373.htm. Accessed on: 19 December 2011.
- ↑ Raz, E.; Zagzag, D.; Saba, L.; Mannelli, L.; Di Paolo, PL.; D'Ambrosio, F.; Knopp, E. (Aug 2012). "Cyst with a mural nodule tumor of the brain.". Cancer Imaging 12: 237-44. doi:10.1102/1470-7330.2012.0028. PMID 22935908.
- ↑ Spears, RC. (Aug 2004). "Colloid cyst headache.". Curr Pain Headache Rep 8 (4): 297-300. PMID 15228889.
- ↑ Kava, MP.; Tullu, MS.; Deshmukh, CT.; Shenoy, A.. "Colloid cyst of the third ventricle: a cause of sudden death in a child.". Indian J Cancer 40 (1): 31-3. PMID 14716130.
- ↑ Shaktawat, SS.; Salman, WD.; Twaij, Z.; Al-Dawoud, A. (2006). "Unexpected death after headache due to a colloid cyst of the third ventricle.". World J Surg Oncol 4: 47. doi:10.1186/1477-7819-4-47. PMID 16867192.
- ↑ 71.0 71.1 71.2 Armao, D.; Castillo, M.; Chen, H.; Kwock, L. (Sep 2000). "Colloid cyst of the third ventricle: imaging-pathologic correlation.". AJNR Am J Neuroradiol 21 (8): 1470-7. PMID 11003281.
- ↑ 72.0 72.1 Turkel, SB.; Miller, CA.; Guttenberg, ME.; Moynes, DR.; Godgman, JE. (Mar 1982). "A clinical pathologic reappraisal of kernicterus.". Pediatrics 69 (3): 267-72. PMID 7063283.
- ↑ 73.0 73.1 URL: http://neuropathology-web.org/chapter3/chapter3eBilirubinencephalopathy.html. Accessed on: 30 May 2012.
- ↑ Hansen, TW.; Hervieux, JF.; Orth, J.; Schmorl, CG.; Baumes, JB. (Aug 2000). "Pioneers in the scientific study of neonatal jaundice and kernicterus.". Pediatrics 106 (2): E15. PMID 10920171.
- ↑ Paksoy, Y.; Koç, H.; Genç, BO.. "Bilateral mesial temporal sclerosis and kernicterus.". J Comput Assist Tomogr 28 (2): 269-72. PMID 15091133.
- ↑ 76.0 76.1 http://www.ninds.nih.gov/disorders/joubert/joubert.htm