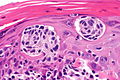
Malignant melanoma
| Malignant melanoma | |
|---|---|
| Diagnosis in short | |
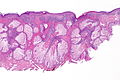
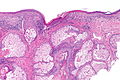
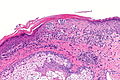
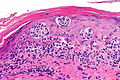
 Malignant melanoma. H&E stain. | |
|
| |
| LM | melanocytic differentiation (e.g. pigment), abnormal architecture, lack of maturation, +/-nuclear atypia - esp. nucleoli, +/-upward scatter of melanocytes, +/-asymmetry of pigmentation |
| LM DDx | dysplastic nevus, Spitz nevus, common nevus (nevoid melanoma), atypical fibroxanthoma, (spindle cell) squamous cell carcinoma, leiomyosarcoma, serous carcinoma, clear cell sarcoma, others |
| Stains | melanin |
| IHC | S-100, Melan A, HMB-45, MITF, tyrosinase |
| EM | melanosomes |
| Molecular | +/-BRAF mutation |
| Site | skin (usu. sun exposed areas), oral mucosa, others |
|
| |
| Syndromes | familial melanoma |
|
| |
| Signs | ABCDE = asymmetrical, border irregular, colour (black), diameter (>6 mm), evolving (growing) |
| Prevalence | common |
| Prognosis | good to very poor (dependent on stage) |
| Clin. DDx | pigmented skin lesions, esp. melanocytic lesions |
| Treatment | wide excision if possible |
| Melanoma in situ | |
|---|---|
| External resources | |
| EHVSC | 10171 |
Malignant melanoma, also melanoma, is an aggressive type of skin cancer that can be diagnostically challenging for pathologists.
It fits into the larger category of melanocytic lesions which includes many benign entities, a number of which can be difficult to distinguish from melanoma.
General
- Known as the great mimicker in pathology; it may look like many things.
Pathologic prognostic factors
Pathologic predictors for a poor prognosis:[1]
- Tumour thickness (Brewslow thickness) > 1 mm.
- Mitotic rate >1/mm^2.
- Ulceration.
- Regression - >75% of tumour.
- Microsatellitosis - nest of tumour cells > 0.05 mm size, separated from primary tumour >=0.3 mm and <= 2 cm.
- In transit metastasis.
- Lymphovascular invasion.
- Perineural invasion.
- Lack of tumour infiltrating lymphocytes (TILs).[citation needed]
Clinical
Serologic predictors of a poor prognosis:
- Lactate dehydrogenase (LDH) > 200-225 U/L.
- Alumin < 35 g/L.
Epidemiology:
- Strong association with sun exposure.
- Typically Caucasians.
Gross
Memory device ABCDE:[4]
- Asymmetric.
- Borders (irregular).
- Colour (black - variable in lesion).
- Diameter (larger than 6 mm).
- Evolving (change with time).
Microscopic
Metastatic/non-skin
Features (non-skin):
- Classic appearance of melanoma:
- Loosely cohesive; mix of small nests of cells, single cells.
- Nests often have clefting with surrounding tissue.
- Mix of spindle cells and epithelioid cells:
- +/-Occasional large binucleated cells.
- Cytoplasm with brown pigment (melanin).
- Prominent (large) red nucleoli (like in serous carcinoma of the ovary).
- Marked nuclear pleomorphism - variation in cell size, shape & staining (like in serous carcinoma of the ovary).
- Nuclear pseudoinclusions (like in papillary thyroid carcinoma).
- Loosely cohesive; mix of small nests of cells, single cells.
Notes:
- Can look almost like anything.
- Like it is said that sarcoidosis is in every internal medicine DDx... melanoma is every pathologic DDx
- May have no nuclear atypia.
- Diagnosis is based on architecture (upward spread in the epidermis, single cells, asymmetry).
DDx
- Carcinoma.
- Serous carcinoma - both serous carcinoma and melanoma have a large nucleolus.
- Sarcoma - as may have spindle cells.
- Clear cell sarcoma (AKA melanoma of the soft parts).
- Metaplastic carcinoma.
- Spindle cell squamous carcinoma.
- Epithelioid angiosarcoma.
- Lymphoma.
- Nodal nevus - benign nevus in lymph node.
- Other (benign) melanocytic lesions.
Images:
Skin
Features (skin):
- Melanocytic differentiation:
- Pigmentation (melanin).
- Nuclear pseudoinclusion.
- Gray cytoplasm.
- Clear (artefactual) halo around cells.
- Architecture:
- Sheeting - diagnostic.
- Asymmetry of architecture - as judged from low power magnification.
- Lack of maturation - see below.
- +/-Nuclear atypia - esp. nucleoli.
- May be seen in a Spitz nevus.
- +/-Upward scatter of melanocytes AKA intraepidermal ascent - "cannonball" appearance.
- No diagnostic significance in the following cases:
- Acral sites - see: Acral nevus.
- Histologic evidence of trauma.
- Thick dense stratum corneum.
- No diagnostic significance in the following cases:
- +/-Asymmetry of pigmentation.
Maturation - with depth:
- Cells get smaller.
- Mitoses decrease.
- Pigmentation decreases.
- Nests get smaller.
Memory device CMPA: Cells, Mitoses, Pigment, Aggregates of cells (nests).
DDx:
- Epithelioid cell forms:
- Spitz nevus - especially difficult.
- Key differences: maturation and symmetry.
- Melanocytic nevus - especially:
- Spitz nevus - especially difficult.
- Spindle cell forms:
- Spindle cell squamous carcinoma.
- Atypical fibroxanthoma.
- Leiomyosarcoma.
- Dermal scar.
- Blue nevus.
Images
www
MIS
Regression of melanoma
General
- Complete regression without metastases estimated to be 10-20%.[5]
- Common ~25% of cases.[5]
- Complete regression and partial regression >75% of the lesion are a poor prognostic feature.[6]
Note:
- Melanocytic lesions in general, not only melanoma, may regress.[7][8]
Microscopic
Features - all required:
- No melanocytes.
- Melanophages.
- Fibrosis.
- Thinned epidermis.
- Telangiectatic vessels.
- Lymphocytes.
Metastatic versus primary
Primary lesions should have:
- Epidermal involvement.
Metastatic lesions classically have:
- Tumour angiotropism (tumours cells cluster around vessels).
- Intravascular invasion.
- No epidermal component.
Note:
- Histology is not definitive for metastatic melanoma vs. primary melanoma; epidermal involvement may be seen in mets.
- IHC (like histology) is not definitive.[9]
- History/clinical is important for differentiation.
Margin assessment
General
- Adequate distance dependent on tumour stage - see surgical margin article.
- Margin assessment is notoriously difficult as there are numerous mimics of melanoma in situ:[10]
- Melanocytic hyperplasia (considered to be on a continuum with melanoma) may be due to:
- Light exposure.
- Peritumoral-effect.
- Previous biopsy.
- Solar lentigo.
- Lichenoid reactions.
- Melanocytic hyperplasia (considered to be on a continuum with melanoma) may be due to:
Microscopic
- Pagetoid spread of melanocytes.
- Junctional or intraepidermal melanocytic nests.
- Three of more contiguous melanocytes in the basal layer.
- Increased numbers of basal melanocytes ( > 25 melanocytes / 0.5 mm of basal layer).
- Marked cytologic atypia - multinucleated cells.
- Adenxal involvement.
Lame mnemonic MARGIN:
- Marked cytologic atypia.
- Adnexal involvement.
- Row of melanocytes.
- Gravity defying melanocytes (Pagetoid spread).
- Increased basal melanocytes.
- Nests of melanocytes.
Assessment/reporting of margins
- There is no general agreement on how to report margins in melanoma.
It is suggested that one should:
- Try to tease apart melanoma cells from benign melanocytes.
- Use the MARGIN mnemonic above.
- Melanocytes with nuclear atypia = melanoma cells.
- Use the MARGIN mnemonic above.
- Report the clearance of the nearest melanoma cell to the margin.
- Positive margin = melanoma cell is touching ink.
- Very close is reported as "clearance < 0.1 mm".
- Use immunostains to assist the assessment of difficult cases:
- MiTF is considered the preferred marker.
- MART-1 (Melan A) is considered to overestimate melanocytes; it should not be used.[11]
- S-100 also marks follicular dendritic cells; it is not a preferred marker.
Breslow thickness
- AKA maximum tumour thickness.
- Depth measured from stratum granulosum to deepest intradermal tumour cell - predictive of survival.[12]
Tumour stage
Melanoma staging is based primarily on the Breslow thickness:[13][14]
- pT1 ≤ 1.0 mm.
- pT1a: ≤ 0.8 mm, no ulceration.
- pT1b: ulceration present or 0.8 mm < thickness ≤ 1.0 mm (with or without ulceration).
- pT2 1.01 mm to 2.0 mm.
- pT2a: no ulceration.
- pT2b: ulceration present.
- pT3 2.01 mm to 4.0 mm.
- pT3a: no ulceration.
- pT3b: ulceration present.
- pT4 >4.0 mm.
- pT4a: no ulceration.
- pT4b: ulceration present.
Clark level
- AKA anatomic level.
- Not as reproducible as Breslow thickness. It is not used for this reason.
Anatomic level - definition:
- I = epidermis only (AKA melanoma in situ).
- II = extends into papillary dermis but does not fill or expand.
- III = fills and expands papillary dermis.
- IV = extends into reticular dermis.
- V = extends into subdermis.
Subtypes
| Subtype name | Key feature | Microscopic additional | DDx | Image | Notes/other |
|---|---|---|---|---|---|
| Melanoma in situ | confined to epidermis, nuclear atypia | melanocyte enlargement, nuclear hyperchromasia, +/- melanocytes above suprapapillary plate (above basal layer) = "Pagetoid spread" | melanocytic hyperplasia, pagetoid Spitz nevus, dysplastic nevus[15] | (upmc.edu), (WC) | lentigo maligna (LM) is melanoma in situ[16] on sun damaged skin; LM should not be confused with lentigo maligna melanoma (LMM) |
| Malignant melanoma - superficial spreading type | atypical melanocytes at all levels of epidermis + dermis | atypical dermal melanocytes single, in cluster or sheets | compound melanocytic nevus | Image? | Notes/other? |
| Malignant melanoma - lentiginous type | atypical melanocytes prominent along basal keratinocytes + in dermis | nuclear atypia | melanoma in situ | Image? | lentigo maligna melanoma (LMM) = lentiginous malignant melanoma with sun damage[citation needed] |
| Malignant melanoma - nodular type | dermal large nodule/sheet | nuclear atypia; may not be prominent in epidermis | metastatic melanoma | Image? | Notes/other? |
| Malignant melanoma - desmoplastic-neurotropic type AKA desmoplastic melanoma | large atypical spindle cells, between collagen | predominantly dermal, +/-lymphocytes (nodules or infiltrating)[17] | pleomorphic undifferentiated sarcoma (MFH), scar, dermatofibroma, DFSP, leiomyosarcoma, desmoplastic Spitz nevus, sclerosing blue nevus | (upmc.edu) | IHC: rarely S100-, generally Melan A- & HMB-45-; subdivided into mixed desmoplastic melanoma and pure desmoplastic melanoma |
| Malignant melanoma - nevoid type | prominent nucleoli, deep mitoses - high power diagnosis | mimics nevus at low power; "push" elastic fibers downward (unlike benign nevi) | (benign) nevus | Image? | deep HMB-45+ |
| Malignant melanoma - spitzoid type | nested pattern, nuclear atypia, no maturation (large deep cells) | NC ratio increased (vs. Spitz) | Spitz nevus | Image? | Notes/other? |
Subtypes in short
| Subtype name | Key feature |
|---|---|
| in situ | confined to epidermis, unlike all others |
| superficial spreading | above basal layer |
| lentiginous | along basal keratinocytes |
| nodular | nodular dermal lesion |
| desmoplastic-neurotropic | atypical dermal spindle cells |
| nevoid | nevus-like at low power |
| spitzoid | mimics Spitz nevus (at DE junction) |
Electron microscopy
Image(s):
Stains
- Fontana-Masson stain, stains melanin.[18]
- May be useful to differentiate melanin from other brown stuff (e.g. lipofuscin, hemosiderin).
IHC
Standard panel
- S-100 +ve.
- Negative staining pretty much excludes the diagnosis.
- HMB-45 +ve -- especially deep, often patchy.
- Melan A (MART-1) +ve.
Notes:
- The standard panel above (S100, HMB-45, MART-1) is also positive in other lesions, e.g. cellular blue nevus.
- Melan A tends to overestimate the number of melanocytes.[11]
- Counting the cell bodies may give an accurate result.[citation needed]
- S-100 marks melanocytes and follicular dendritic cells.
Threshold panel
When one it sure it is melanocytic... but unsure whether it is melanoma:
- HMB-45 +ve deep melanocytes.
- In benign lesions deep (mature) melanocytes are negative.
- Ki-67.
Sentinel lymph node panel
Three sets of the following (12 slides in total):
- H&E.
- S-100.
- MART-1.
- HMB-45.
Notes:
- Positive in approximately 20% of cases - based on one series.[19]
- Strongly dependent on T-stage (T1 ~5%, T2 ~11%, T3 ~28%, T4 ~47%).
Others
- SOX10 +ve -- useful for differentiate from excision scar.[20]
- SOX10 = pan-schwannian and melanocytic marker.
- CD99 +ve.
- Melanoma cocktail (HMB-45, MART-1).[21]
- Microphthalmia transcription factor (MITF) - easy to interpret as it is a nuclear stain.[22][23]
- Tyrosinase.[24]
- WT1 usually +ve[25] - not commonly used.
Molecular
- Commonly have BRAF mutations.[26]
- Desmoplastic melanoma has the highest number of mutations (62 per megabase).[27]
- The high number of (C>T) transitions suggest UV radiation as main cause.
- Approx. 15% of the cases have NFKBIE amplifications.
Sign out
Melanoma in situ
Skin Lesion, Left Upper Back, Re-excision: - Melanoma in situ, completely excised. -- Surgical clearance 8 millimetres. - Dermal scar. - Solar elastosis. Comment: The case was partially reviewed with Dr. X; he agrees melanoma in situ is present.
Block letters
SKIN LESION, MID-MIDDLE BACK, PUNCH BIOPSY: - MELANOMA IN SITU, NEAREST (LATERAL) MARGIN APPROXIMATELY 1 MM -- WIDE RE-EXCISION SHOULD BE DONE. COMMENT: The lesion is characterized by mild nuclear atypia and marked architectural complexity. It has lamellar fibrosis and multiple foci of complex rete ridge bridging and pagetoid spread of melanocytes. Mitotic activity is seen focally.
SKIN LESION, MID BACK, EXCISION: - LENTIGO MALIGNA (SOLAR ELASTOSIS AND MELANOMA IN SITU), MARGIN CLEARANCE < 0.1 MM. COMMENT: This lesion should be re-excised.
SKIN LESION, MID BACK, EXCISION: - MELANOMA IN SITU AND SOLAR ELASTOSIS (LENTIGO MALIGNA), MARGIN CLEARANCE 2 MM. COMMENT: The presence of melanoma in situ is confirmed with immunostaining (HMB-45, MITF). This lesion should be re-excised.
At least MIS
LEFT THUMB NAIL, AVULSION AND NAIL MATRIX BIOPSY: - AT LEAST MALIGNANT MELANOMA IN SITU. COMMENT: The quantity of diagnostic material is suboptimal. The limited number of lesional cells stain as follows: POSITIVE: S-100, HMB-45, MITF, Melan A. An internal review confirms the impression of at least melanoma in situ.
Micro
The sections show hair-bearing skin with atypical melanocytes confined to the epidermis. The melanocytes scatter upwards (focally), have confluent growth and nucleoli, and involve the adnexal structures. Occasional large multi-nucleated melanocytes, with their nuclei arranged around the cell periphery, are present. Mitotic activity is not apparent. Extensive solar elastosis is present.
The lesion is very close to the margin (<0.1 mm clearance).
Alternate
The sections show skin with pigmented atypical melanocytes confined to the epidermis. The melanocytes scatter upwards, and have focal confluent growth and nucleoli. Mitotic activity is not apparent. Solar elastosis is present in the background. The lesion extends to the edge of the tissue; it is incompletely excised.
Invasive melanoma
SKIN LESION, LEFT LOWER BACK, SHAVE BIOPSY: - INVASIVE MALIGNANT MELANOMA. -- AT LEAST pT3a. -- 6 MITOSES/MM*MM. -- DEEP AND LATERAL MARGINS POSITIVE, WIDE RE-EXCISION SHOULD BE DONE. -- PLEASE SEE TUMOUR SUMMARY. -- PLEASE SEE COMMENT. COMMENT: The morphologic impression is confirmed by immunostains; the tumour is POSITIVE for S-100, HMB-45, and MART-1.
See also
References
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/SkinMelanoma_11protocol.pdf. Accessed on: 29 March 2012.
- ↑ Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 362 Q49. ISBN 978-1416025887.
- ↑ Byrd-Miles, K.; Toombs, EL.; Peck, GL. (Jan 2007). "Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians.". J Drugs Dermatol 6 (1): 10-6. PMID 17373156.
- ↑ Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 466. ISBN 978-0443066542.
- ↑ 5.0 5.1 Printz, C. (Jul 2001). "Spontaneous regression of melanoma may offer insight into cancer immunology.". J Natl Cancer Inst 93 (14): 1047-8. PMID 11459861.
- ↑ Crowson, AN.; Magro, CM.; Mihm, MC. (Feb 2006). "Prognosticators of melanoma, the melanoma report, and the sentinel lymph node.". Mod Pathol 19 Suppl 2: S71-87. doi:10.1038/modpathol.3800517. PMID 16446717.
- ↑ Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 476. ISBN 978-0443066542.
- ↑ Speeckaert, R.; van Geel, N.; Vermaelen, KV.; Lambert, J.; Van Gele, M.; Speeckaert, MM.; Brochez, L. (Apr 2011). "Immune reactions in benign and malignant melanocytic lesions: lessons for immunotherapy.". Pigment Cell Melanoma Res 24 (2): 334-44. doi:10.1111/j.1755-148X.2010.00799.x. PMID 21029398.
- ↑ Guerriere-Kovach, PM.; Hunt, EL.; Patterson, JW.; Glembocki, DJ.; English, JC.; Wick, MR. (Jul 2004). "Primary melanoma of the skin and cutaneous melanomatous metastases: comparative histologic features and immunophenotypes.". Am J Clin Pathol 122 (1): 70-7. doi:10.1309/FUQH-92B0-3902-5LHG. PMID 15272532.
- ↑ 10.0 10.1 Trotter, MJ. (Jun 2011). "Melanoma margin assessment.". Clin Lab Med 31 (2): 289-300. doi:10.1016/j.cll.2011.03.006. PMID 21549242.
- ↑ 11.0 11.1 Kim, J.; Taube, JM.; McCalmont, TH.; Glusac, EJ. (Oct 2011). "Quantitative comparison of MiTF, Melan-A, HMB-45 and Mel-5 in solar lentigines and melanoma in situ.". J Cutan Pathol 38 (10): 775-9. doi:10.1111/j.1600-0560.2011.01763.x. PMID 21797920.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 595. ISBN 978-1416054542.
- ↑ Nowecki, ZI.; Rutkowski, P.; Michej, W. (Aug 2008). "The survival benefit to patients with positive sentinel node melanoma after completion lymph node dissection may be limited to the subgroup with a primary lesion Breslow thickness greater than 1.0 and less than or equal to 4 mm (pT2-pT3).". Ann Surg Oncol 15 (8): 2223-34. doi:10.1245/s10434-008-9965-3. PMID 18506535.
- ↑ URL: https://documents.cap.org/protocols/Skin.Melanoma.Bx_4.3.0.1.REL_CAPCP.pdf. Accessed on: 14 September 2021.
- ↑ Farrahi, F.; Egbert, BM.; Swetter, SM. (Jul 2005). "Histologic similarities between lentigo maligna and dysplastic nevus: importance of clinicopathologic distinction.". J Cutan Pathol 32 (6): 405-12. doi:10.1111/j.0303-6987.2005.00355.x. PMID 15953373.
- ↑ McKenna, JK.; Florell, SR.; Goldman, GD.; Bowen, GM. (Apr 2006). "Lentigo maligna/lentigo maligna melanoma: current state of diagnosis and treatment.". Dermatol Surg 32 (4): 493-504. doi:10.1111/j.1524-4725.2006.32102.x. PMID 16681656.
- ↑ URL: http://path.upmc.edu/cases/case378/dx.html. Accessed on: 1 June 2012.
- ↑ URL: http://education.vetmed.vt.edu/curriculum/VM8054/labs/Lab2/Examples/exfontana.htm. Accessed on: 5 May 2010.
- ↑ Teixeira V, Vieira R, Coutinho I, et al. (2013). "Prediction of sentinel node status and clinical outcome in a melanoma centre". J Skin Cancer 2013: 904701. doi:10.1155/2013/904701. PMC 3886376. PMID 24455276. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3886376/.
- ↑ Ramos-Herberth FI, Karamchandani J, Kim J, Dadras SS (September 2010). "SOX10 immunostaining distinguishes desmoplastic melanoma from excision scar". J. Cutan. Pathol. 37 (9): 944–52. doi:10.1111/j.1600-0560.2010.01568.x. PMID 20653825.
- ↑ Jani P, Chetty R, Ghazarian DM (April 2008). "An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature". Am J Dermatopathol 30 (2): 174–7. doi:10.1097/DAD.0b013e318165b8fe. PMID 18360125.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 156845
- ↑ Levy, C.; Khaled, M.; Fisher, DE. (Sep 2006). "MITF: master regulator of melanocyte development and melanoma oncogene.". Trends Mol Med 12 (9): 406-14. doi:10.1016/j.molmed.2006.07.008. PMID 16899407.
- ↑ Roma, AA.; Magi-Galluzzi, C.; Zhou, M. (Jan 2007). "Differential expression of melanocytic markers in myoid, lipomatous, and vascular components of renal angiomyolipomas.". Arch Pathol Lab Med 131 (1): 122-5. doi:10.1043/1543-2165(2007)131[122:DEOMMI]2.0.CO;2. PMID 17227112.
- ↑ Wilsher M, Cheerala B (November 2007). "WT1 as a complementary marker of malignant melanoma: an immunohistochemical study of whole sections". Histopathology 51 (5): 605–10. doi:10.1111/j.1365-2559.2007.02843.x. PMID 17927581.
- ↑ Brose, MS.; Volpe, P.; Feldman, M.; Kumar, M.; Rishi, I.; Gerrero, R.; Einhorn, E.; Herlyn, M. et al. (Dec 2002). "BRAF and RAS mutations in human lung cancer and melanoma.". Cancer Res 62 (23): 6997-7000. PMID 12460918.
- ↑ Shain, AH.; Garrido, M.; Botton, T.; Talevich, E.; Yeh, I.; Sanborn, JZ.; Chung, J.; Wang, NJ. et al. (Oct 2015). "Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway.". Nat Genet 47 (10): 1194-9. doi:10.1038/ng.3382. PMID 26343386.