Difference between revisions of "Medical lung diseases"
(→Pulmonary nodular lymphoid hyperplasia: split out) |
|||
| (8 intermediate revisions by the same user not shown) | |||
| Line 64: | Line 64: | ||
==Constrictive bronchiolitis== | ==Constrictive bronchiolitis== | ||
*[[AKA]] ''bronchiolitis obliterans'', [[AKA]] ''obliterative broncholitis'',<ref name=pmid16493150/> [[AKA]] bronchiolitis obliterans syndrome (BOS).<ref name=pmid18707652>{{Cite journal | last1 = Sato | first1 = M. | last2 = Keshavjee | first2 = S. | title = Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. | journal = Semin Thorac Cardiovasc Surg | volume = 20 | issue = 2 | pages = 173-82 | month = | year = 2008 | doi = 10.1053/j.semtcvs.2008.05.002 | PMID = 18707652 }}</ref> | *[[AKA]] ''bronchiolitis obliterans'', [[AKA]] ''obliterative broncholitis'',<ref name=pmid16493150/> [[AKA]] bronchiolitis obliterans syndrome (BOS).<ref name=pmid18707652>{{Cite journal | last1 = Sato | first1 = M. | last2 = Keshavjee | first2 = S. | title = Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. | journal = Semin Thorac Cardiovasc Surg | volume = 20 | issue = 2 | pages = 173-82 | month = | year = 2008 | doi = 10.1053/j.semtcvs.2008.05.002 | PMID = 18707652 }}</ref> | ||
{{Main|Constrictive bronchiolitis}} | |||
==Diffuse lung diseases== | ==Diffuse lung diseases== | ||
| Line 183: | Line 148: | ||
|stains to exclude lymphoma; germinal centres do not exclude [[lymphoma]] | |stains to exclude lymphoma; germinal centres do not exclude [[lymphoma]] | ||
|- | |- | ||
|Lymphoma (BALToma) | |Lymphoma ([[BALToma]]) | ||
|abundant lymphoid cells usu. in nodules | |abundant lymphoid cells usu. in nodules | ||
|nodules / interstitial pattern | |nodules / interstitial pattern | ||
| Line 198: | Line 163: | ||
==Pulmonary nodular lymphoid hyperplasia== | ==Pulmonary nodular lymphoid hyperplasia== | ||
{{Main|Pulmonary nodular lymphoid hyperplasia}} | {{Main|Pulmonary nodular lymphoid hyperplasia}} | ||
==Lymphoma of the lung== | |||
{{Main|Lymphoma of the lung}} | |||
=[[Smoking]] associated disease= | =[[Smoking]] associated disease= | ||
{{Main|Smoking}} | {{Main|Smoking}} | ||
*RB | *Respiratory bronchiolitis (RB). | ||
* | *[[Respiratory bronchiolitis interstitial lung disease]] (RBILD). | ||
* | *[[Desquamative interstitial pneumonia]] (DIP). | ||
*Eosinophilic granuloma (of lung) - [[AKA]] pulmonary langerhans cell histiocytosis. | *Eosinophilic granuloma (of lung) - [[AKA]] [[pulmonary langerhans cell histiocytosis]]. | ||
*[[Smoking-related interstitial fibrosis]] (SRIF). | |||
All of the above are | All of the above are associated with smoking. RBILD & DIP are considered by many to be on a continuum, i.e. RBILD is early DIP. | ||
==Respiratory bronchiolitis== | ==Respiratory bronchiolitis== | ||
| Line 237: | Line 206: | ||
*Talc granulomatosis. | *Talc granulomatosis. | ||
*Sarcoidosis. | *Sarcoidosis. | ||
*Wegener granulomatosis. | *[[Granulomatosis with polyangiitis]] (Wegener granulomatosis). | ||
==Sarcoidosis== | ==Sarcoidosis== | ||
| Line 338: | Line 307: | ||
=Eosinophilic pneumonia= | =Eosinophilic pneumonia= | ||
Specific entities:<ref name=emedicine301070>[http://emedicine.medscape.com/article/301070-overview http://emedicine.medscape.com/article/301070-overview]</ref> | Specific entities:<ref name=emedicine301070>[http://emedicine.medscape.com/article/301070-overview http://emedicine.medscape.com/article/301070-overview]</ref> | ||
*[[Churg-Strauss syndrome | *[[Eosinophilic granulomatosis with polyangiitis]] (Churg-Strauss syndrome). | ||
*Acute eosinophilic pneumonia. | *Acute eosinophilic pneumonia. | ||
*Chronic eosinophilic pneumonia. | *Chronic eosinophilic pneumonia. | ||
Latest revision as of 14:54, 23 March 2017
The medical lung diseases are a huge topic. Most pathologists have little to do with 'em. They are the domain of respirology. An introduction to lung pathology is in the lung article, along with a general approach. Interstitial lung disease is dealt with in the diffuse lung diseases article.
Infectious pneumonia
Includes:
Asthma
Emphysema
- Chronic obstructive pulmonary disease, abbreviated COPD, is dealt with in the emphysema article.
Chronic bronchitis
General
- Often seen together with emphysema with which it is lumped together with in the term COPD.
- It's a clinical diagnosis - criteria:[1]
- Cough with sputum for thee months in at least two consecutive years.
- No other cause identified.
Clinical:[1]
- Blue bloater (carbon dioxide retainers)
- Develop cor pulmonale.
Microscopic
Features:[1]
- Mucous gland hypertrophy + mucinous secretions in airway.
- Goblet cell metaplasia.
- Bronchiolar inflammation and fibrosis.
Pulmonary edema
General
- Seen in a number of conditions, e.g. congestive heart failure.
Gross
Features - autopsy:
- Bubbles - when squeezed (due to surfactant).
- Heavy.
Microscopic
Features:[2]
- Dilated capillaries.
- Blood in airspace, focal.
- Plasma proteins in airspace - light pink acellular junk.
- +/-Hemosiderin-laden macrophages (known as heart failure cells in this context).
DDx:
- Pulmonary alveolar proteinosis.
- Pulmonary hemorrhage - abundant blood.
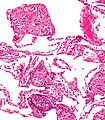
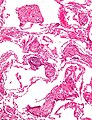
Images:
Bronchiectasis
Pulmonary hemorrhage
Constrictive bronchiolitis
- AKA bronchiolitis obliterans, AKA obliterative broncholitis,[3] AKA bronchiolitis obliterans syndrome (BOS).[4]
Diffuse lung diseases
These are also known as idiopathic interstitial pneumonias.
Fibrosis
Histomorphological classification
- Hyaline membranes - glassy pink material lining airways & alveoli.
- Microscopic honeycombing - "holes" in the lung.
- Bronchiolization - ciliated (respiratory) epithelium in distal airway.
- Uniform alveolar septal thickening - septae look similar at low power.
- Peripheral lobular fibrosis - septae thickening peripheral, HRCT shows: irregular peripheral reticular opacities.[5]
- Reticular = net-like.[6]
- Siderophages in alveoli - macrophages with hemosiderin the alveoli.
- Fibrinous pleuritis - peripheral only (based on imaging).
- Granulomata, non-necrotizing.
- Abundance of vacuolated cells.
- Chronic inflammation.
- Bronchiolocentric scarring - fibrosis concentrated around airway/assoc. with airway.
Radiologic/gross pathologic DDx by location
Causes of lower lung fibrosis BAD RASH:[7]
- Bronchiolitis obliterans organizing pneumonia (BOOP).
- Asbestosis.
- Drugs (nitrofurantoin, hydralazine, isoniazid (INH), amiodarone).
- Rheumatologic disease.
- Aspiration.
- Scleroderma.
- Hamman-Rich syndrome (really should be -- idiopathic pulmonary fibrosis).
Note:
- Hamman-Rich syndrome is another name for acute interstitial pneumonia.[8]
Causes of upper lung fibrosis FASSTEN:[7]
- Farmer's lung.
- Ankylosing spondylitis.
- Sarcoidosis.
- Silicosis.
- Tuberculosis (miliary).
- Eosinophilic granuloma.
- Neurofibromatosis.
Prognosis
- The pattern and severity of fibrosis seems to be the most important factors prognostically - more important than the underlying cause (ILD, CVD, drug reaction etc.).[9][10]
Patterns of fibrosis:
- "Linear" - follows alveolar walls, no architectural distortion.
- UIP-like (honeycombing).
Disease with fibrosis
There are many of 'em.
Fibrosing pleuritis
Lymphocytic lesions of the lung
| Diagnosis | Key histologic feature | Radiology | Other diagnostic |
|---|---|---|---|
| Lymphocytic interstitial pneumonia | interstitial lymphoid cells, usu. no nodules | interstitial pattern | |
| Follicular bronchiolitis/bronchitis | lymphoid cell around bronchioles / bronchus, normal parenchyma | interstitial pattern | |
| Nodular lymphoid hyperplasia | abundant lymphoid cells in nodules | nodules /interstitial pattern | stains to exclude lymphoma; germinal centres do not exclude lymphoma |
| Lymphoma (BALToma) | abundant lymphoid cells usu. in nodules | nodules / interstitial pattern | may require stains to prove, germinal centres may be present |
Lymphocytic interstitial pneumonia
Follicular bronchitis/bronchiolitis
Pulmonary nodular lymphoid hyperplasia
Lymphoma of the lung
Smoking associated disease
- Respiratory bronchiolitis (RB).
- Respiratory bronchiolitis interstitial lung disease (RBILD).
- Desquamative interstitial pneumonia (DIP).
- Eosinophilic granuloma (of lung) - AKA pulmonary langerhans cell histiocytosis.
- Smoking-related interstitial fibrosis (SRIF).
All of the above are associated with smoking. RBILD & DIP are considered by many to be on a continuum, i.e. RBILD is early DIP.
Respiratory bronchiolitis
- Diagnosis is based on clinical criteria.
Microscopic
Features:
- Inflammation.
- No interstitial lung disease, i.e. no fibrosis.
Respiratory bronchiolitis interstitial lung disease
Desquamative interstitial pneumonia
Pulmonary Langerhans cell histiocytosis
- AKA eosinophilic granuloma of the lung.
Granulomatous lung disease
- See: Granulomas for an introduction to the general topic.
Most common:
- Infectious - mycobacterial and fungal.[11]
Noninfectious causes:[11]
- Aspiration pneumonia.
- Hypersensitivity pneumonitis.
- Hot tub lung.
- Talc granulomatosis.
- Sarcoidosis.
- Granulomatosis with polyangiitis (Wegener granulomatosis).
Sarcoidosis
General
- Diagnosis of exclusion - infection must be excluded.
- Radiologic differential diagnosis includes carcinomatosis.[12]
Microscopic
Features:
- Granulomata, well-formed, non-necrotizing.
Image(s):
Pulmonary talcosis
General
- Associated with herion use.[13]
- Seen in drug users that intravenously inject crushed pills intended to be taken PO.[14]
- X-ray findings similar to asbestosis.
Microscopic
Features:
- Granulomas with foreign material.
- Foreign material often polarizes.
Images
www:
Miscellaneous diseases
Pneumoconioses
Pneumocytoma
Lymphangioleiomyomatosis
- Abbreviated LAM.
- AKA lymphangiomyomatosis.
Pulmonary alveolar proteinosis
- Abbreviated PAP.
Diffuse panbronchiolitis
- Abbreviated DPB.
Pulmonary amyloidosis
General
- Rare.[16]
Microscopic
Features:
- Interstitial cotton candy-like material - see amyloidosis.
DDx:
Images:
Drug reactions
- Effects are often non-specific.
Website: http://www.pneumotox.com
Pulmonary hypertension
General classification:
- Primary, i.e. primary pulmonary hypertension, or
- Secondary, e.g. due to congenital heart disease (like ventricular septal defect), interstitial pulmonary fibrosis.
Non-secondary pulmonary hypertension
Causes:[17]
- Primary pulmonary hypertension.
- Pulmonary embolic disease (thromboembolism, and non-thrombotic embolism).
- Pulmonary capillary haemangiomatosis (PCH).
- Pulmonary veno-occlusive disease (PVOD).
Severity
- Heath-Edwards classification - see pulmonary hypertension.
Eosinophilic pneumonia
Specific entities:[18]
- Eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome).
- Acute eosinophilic pneumonia.
- Chronic eosinophilic pneumonia.
- Eosinophilic granuloma (pulmonary histiocytosis X, Langerhans cell granulomatosis).
Entities which may have eosinophilia as prominent feature:
- AIDS.
- Lymphoma.
- Collagen vascular disease.
Churg-Strauss syndrome
Microscopic
Features:
- Small vessel vasculitis.
- Abundant eosinophils.
- Granulomas.
Eosinophilic pleural effusions
Causes - mnemonic I'M PAID:[20]
- Infection, e.g. tuberculosis.
- Malignancy - uncommon.
- Pulmonary emboli.
- Asbestos exposure.
- Inflammatory diseases.
- Drug reactions.
Lung transplant pathology
This subspecialty is dealt with in its own article.
See also
References
- ↑ 1.0 1.1 1.2 Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 370. ISBN 978-1416054542.
- ↑ Klatt, Edward C. (2006). Robbins and Cotran Atlas of Pathology (1st ed.). Saunders. pp. 102. ISBN 978-1416002741.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid16493150 - ↑ Sato, M.; Keshavjee, S. (2008). "Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling.". Semin Thorac Cardiovasc Surg 20 (2): 173-82. doi:10.1053/j.semtcvs.2008.05.002. PMID 18707652.
- ↑ http://www.rsna.org/Publications/rsnanews/may06/jrnl_may06.cfm
- ↑ http://dictionary.reference.com/browse/reticular
- ↑ 7.0 7.1 Yeung, J.C.; Leonard, Blair J. N. (2005). The Toronto Notes 2005 - Review for the MCCQE and Comprehensive Medical Reference (2005 ed.). The Toronto Notes Inc. for Medical Students Inc.. pp. R13. ISBN 978-0968592854.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 90. ISBN 978-0781765275.
- ↑ Bjoraker JA, Ryu JH, Edwin MK, et al. (January 1998). "Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis". Am. J. Respir. Crit. Care Med. 157 (1): 199-203. PMID 9445300. http://ajrccm.atsjournals.org/cgi/content/full/157/1/199.
- ↑ AC UBC S.425.
- ↑ 11.0 11.1 Mukhopadhyay S, Gal AA (May 2010). "Granulomatous lung disease: an approach to the differential diagnosis". Arch. Pathol. Lab. Med. 134 (5): 667–90. PMID 20441499.
- ↑ URL: http://www.radiologyassistant.nl/en/46b480a6e4bdc. Accessed on: 23 May 2010.
- ↑ Davis, LL. (Dec 1983). "Pulmonary "mainline" granulomatosis: talcosis secondary to intravenous heroin abuse with characteristic x-ray findings of asbestosis.". J Natl Med Assoc 75 (12): 1225–8. PMC 2561715. PMID 6655726. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2561715/.
- ↑ Marchiori, E.; Lourenço, S.; Gasparetto, TD.; Zanetti, G.; Mano, CM.; Nobre, LF. (Apr 2010). "Pulmonary talcosis: imaging findings.". Lung 188 (2): 165-71. doi:10.1007/s00408-010-9230-y. PMID 20155272.
- ↑ Chan, KW.; Gibbs, AR.; Lo, WS.; Newman, GR. (Jun 1982). "Benign sclerosing pneumocytoma of lung (sclerosing haemangioma).". Thorax 37 (6): 404-12. PMID 6291188.
- ↑ Hagmeyer, L.; Stieglitz, S.; Röcken, C.; Randerath, W. (Jun 2012). "[Amyloidosis in Pneumology.]". Pneumologie. doi:10.1055/s-0032-1309811. PMID 22692971.
- ↑ Bush A (December 2000). "Pulmonary hypertensive diseases". Paediatr Respir Rev 1 (4): 361-7. doi:10.1053/prrv.2000.0077. PMID 16263465.
- ↑ http://emedicine.medscape.com/article/301070-overview
- ↑ Matthai, SM.; Kini, U. (Feb 2003). "Diagnostic value of eosinophils in pleural effusion: a prospective study of 26 cases.". Diagn Cytopathol 28 (2): 96-9. doi:10.1002/dc.10227. PMID 12561030.
- ↑ 20.0 20.1 Kalomenidis, I.; Light, RW. (Jul 2004). "Pathogenesis of the eosinophilic pleural effusions.". Curr Opin Pulm Med 10 (4): 289-93. PMID 15220754.