Difference between revisions of "Fibroblastic/myofibroblastic tumours"
Jensflorian (talk | contribs) (→Hemangiopericytoma: images added) |
(split out) |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 111: | Line 111: | ||
==Elastofibroma== | ==Elastofibroma== | ||
{{Main|Elastofibroma}} | |||
==Nodular fasciitis== | ==Nodular fasciitis== | ||
| Line 289: | Line 271: | ||
===Molecular=== | ===Molecular=== | ||
Characteristic [[translocation]]:<ref>{{Cite journal | last1 = Sheng | first1 = WQ. | last2 = Hisaoka | first2 = M. | last3 = Okamoto | first3 = S. | last4 = Tanaka | first4 = A. | last5 = Meis-Kindblom | first5 = JM. | last6 = Kindblom | first6 = LG. | last7 = Ishida | first7 = T. | last8 = Nojima | first8 = T. | last9 = Hashimoto | first9 = H. | title = Congenital-infantile fibrosarcoma. A clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin-embedded tissues. | journal = Am J Clin Pathol | volume = 115 | issue = 3 | pages = 348-55 | month = Mar | year = 2001 | doi = 10.1309/3H24-E7T7-V37G-AKKQ | PMID = 11242790 }}</ref> | Characteristic [[translocation]]:<ref name=pmid11242790 >{{Cite journal | last1 = Sheng | first1 = WQ. | last2 = Hisaoka | first2 = M. | last3 = Okamoto | first3 = S. | last4 = Tanaka | first4 = A. | last5 = Meis-Kindblom | first5 = JM. | last6 = Kindblom | first6 = LG. | last7 = Ishida | first7 = T. | last8 = Nojima | first8 = T. | last9 = Hashimoto | first9 = H. | title = Congenital-infantile fibrosarcoma. A clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin-embedded tissues. | journal = Am J Clin Pathol | volume = 115 | issue = 3 | pages = 348-55 | month = Mar | year = 2001 | doi = 10.1309/3H24-E7T7-V37G-AKKQ | PMID = 11242790 }}</ref> | ||
*t(12;15)(p13;q25). | *t(12;15)(p13;q25). | ||
**Gene fusion ETV6-NTRK3. | **Gene fusion ETV6-[[NTRK3]]. | ||
***Same translocation in [[mesoblastic nephroma]]. | ***Same translocation in [[mesoblastic nephroma]]. | ||
| Line 390: | Line 372: | ||
Feature:<ref name=Ref_WMSP611>{{Ref WMSP|611}}</ref> | Feature:<ref name=Ref_WMSP611>{{Ref WMSP|611}}</ref> | ||
*Spindle cell lesion. | *Spindle cell lesion. | ||
*Herring bone pattern - '''key feature'''. | *[[Herring bone pattern]] - '''key feature'''. | ||
*Mitoses. | *Mitoses. | ||
| Line 416: | Line 398: | ||
*Should '''not''' be confused with ''[[low-grade fibromyxoid sarcoma]]''.<ref name=pmid8650138>{{Cite journal | last1 = Mentzel | first1 = T. | last2 = Katenkamp | first2 = D. | last3 = Fletcher | first3 = CD. | title = [Low malignancy myxofibrosarcoma versus low malignancy fibromyxoid sarcoma. Distinct entities with similar names but different clinical course]. | journal = Pathologe | volume = 17 | issue = 2 | pages = 116-21 | month = Mar | year = 1996 | doi = | PMID = 8650138 }}</ref> | *Should '''not''' be confused with ''[[low-grade fibromyxoid sarcoma]]''.<ref name=pmid8650138>{{Cite journal | last1 = Mentzel | first1 = T. | last2 = Katenkamp | first2 = D. | last3 = Fletcher | first3 = CD. | title = [Low malignancy myxofibrosarcoma versus low malignancy fibromyxoid sarcoma. Distinct entities with similar names but different clinical course]. | journal = Pathologe | volume = 17 | issue = 2 | pages = 116-21 | month = Mar | year = 1996 | doi = | PMID = 8650138 }}</ref> | ||
*[[AKA]] ''myxoid malignant fibrous histiocytoma'' or ''myxoid MFH''.<ref>{{Cite journal | last1 = Fujimura | first1 = T. | last2 = Okuyama | first2 = R. | last3 = Terui | first3 = T. | last4 = Okuno | first4 = K. | last5 = Masu | first5 = A. | last6 = Masu | first6 = T. | last7 = Chiba | first7 = S. | last8 = Kunii | first8 = T. | last9 = Tagami | first9 = H. | title = Myxofibrosarcoma (myxoid malignant fibrous histiocytoma) showing cutaneous presentation: report of two cases. | journal = J Cutan Pathol | volume = 32 | issue = 7 | pages = 512-5 | month = Aug | year = 2005 | doi = 10.1111/j.0303-6987.2005.00368.x | PMID = 16008697 }}</ref> | *[[AKA]] ''myxoid malignant fibrous histiocytoma'' or ''myxoid MFH''.<ref>{{Cite journal | last1 = Fujimura | first1 = T. | last2 = Okuyama | first2 = R. | last3 = Terui | first3 = T. | last4 = Okuno | first4 = K. | last5 = Masu | first5 = A. | last6 = Masu | first6 = T. | last7 = Chiba | first7 = S. | last8 = Kunii | first8 = T. | last9 = Tagami | first9 = H. | title = Myxofibrosarcoma (myxoid malignant fibrous histiocytoma) showing cutaneous presentation: report of two cases. | journal = J Cutan Pathol | volume = 32 | issue = 7 | pages = 512-5 | month = Aug | year = 2005 | doi = 10.1111/j.0303-6987.2005.00368.x | PMID = 16008697 }}</ref> | ||
{{Main|Myxofibrosarcoma}} | |||
=See also= | =See also= | ||
Latest revision as of 18:58, 24 March 2019
This article covers fibroblastic/myofibroblastic tumours. These tumours fit into the larger category of soft tissue lesions.
List of tumours
Benign
WHO classification:[1]
- Nodular fasciitis.
- Proliferative fasciitis.
- Proliferative myositis.
- Myositis ossificans.
- Ischemic fasciitis.
- Elastofibroma.
- Myofibroma.
- Fibromatosis colli.
- Inclusion body fibromatosis.
- Fibroma of tendon sheath.
- Calcifying aponeurotic fibroma.
- Angiomyofibroblastoma.
- Cellular angiofibroma.
- Nuchal-type fibroma.
- Gardner fibroma.
- Calcifying fibrous tumour.
- Giant cell angiofibroma.
- Fibrous hamartoma of infancy.
- Juvenile hyaline fibromatosis.
- Desmoplastic fibroblastoma.
- Mammary-type myofibroblastoma.
Locally aggressive
WHO classification:[1]
- Superfical fibromatosis.
- Desmoid-type fibromatosis.
- Lipofibromatosis.
Occasionally metastasizing
WHO classification:[2]
- Solitary fibrous tumour.
- Inflammatory myofibroblastic tumour.
- Low-grade myofibroblastic sarcoma.
- Myxoinflammatory fibroblastic sarcoma.
- Infantile fibrosarcoma.
Malignant
WHO classification:[2]
- Adult fibrosarcoma.
- Myxofibrosarcoma.
- Low-grade fibromyxoid sarcoma (hyalinizing spindle cell tumour).
- Sclerosing epithelioid fibrosarcoma.
Non-malignant
Proliferative fasciitis
General
- Benign.
- May mimic a sarcoma.[3]
Clinical:
- Solid subcutaneous nodule.
- Rapid growth.
- May be painful.
Gross
- Classically upper and lower extremities.[3]
- Poorly demarcated.
Microscopic
Features:[4]
- Large polygonal (ganglion-like) and/or spindled cells with:
- Vesicular (clear) nuclei.
- Prominent nucleoli.
- +/-Binucleation.
- Loose myxoid stroma.
- Frequent typical mitoses.
- No atypical mitoses.
DDx:
Images:
- Proliferative fasciitis - several images (virginia.edu).
- Proliferative fasciitis (surgicalpathologyatlas.com).
Proliferative myositis
General
- Benign.
- Possible arise from pericytes.[6]
Microscopic
- Large ganglion-like cells.
- Cells have single prominent nucleolus.
- Spindle cells.
- +/-Binucleation.
- Mitotic activity.
- No atypical mitoses.
Image:
IHC
Features:[6]
- Vimentin +ve.
- SMA +ve.
- Desmin +ve/-ve.
Others:[6]
- Factor XIIIa -ve.
- S100 -ve.
- CAM5.2 -ve.
- NSE -ve.
Elastofibroma
Nodular fasciitis
Desmoid-type fibromatosis
Lipofibromatosis
- AKA infantile subcutaneous fibromatosis.
General
- Childhood.
Microscopic
Features:[8]
- Fibroblastic cells surrounding adipocytes.
Image:
IHC
Features:[8]
- CD34 +ve.
- BCL2 +ve.
- S100 +ve.
- CD99 +ve.
- Actin +ve.
- EMA +ve.
Desmoplastic fibroblastoma
- AKA collagenous fibroma.[9]
- Not to be confused with desmoplastic fibroma.
General
- Benign lesion.
Epidemiology:
Gross
- Classically found in the shoulder region.
DDx - shoulder region:
Microscopic
- Spindle cells or stellate cells without nuclear atypia.
- Acellular stroma with abundant collagen - key feature.
- +/-Myxoid areas.
- +/-Rare mitoses.
DDx:[11]
Images:
IHC
Features:[11]
- Beta-catenin -ve.[13]
- +ve in desmoid-type fibromatosis.
- Desmin -ve.
- S-100 -ve.
- CD34 -ve.
- MSA +ve (focal).
- alpha-SMA +ve (focal).
Molecular
- llq12 breakpoint described as being characteristic -- possibly the FOSL1 gene.[14]
Calcifying fibrous tumour
General
- Rare.
- Benign.
Microscopic
Features:[15]
- Submucosal circumscribed fibrocollagenous nodule.
- Psammomatous calcifications.
- Focal plasma cells at the periphery.
Myofibroma
Cellular angiofibroma
General
- Rare.
- Benign.
- Probably related to spindle cell lipoma and mammary-type myofibroblastoma.[16]
- Predominantly female.
Gross
Features:[16]
- Superficial.
- Well-circumscribed.
Classic location:
- Vulva.[16]
Microscopic
Features:[16]
- Spindle cell lesion.
- Many small-to-medium blood vessls.
IHC
Features:[16]
- CD34 ~50% of cases.
- SMA ~41% of cases.
- CD99 -ve.
- EMA -ve.
Occasionally metastasizing
Inflammatory myofibroblastic tumour
- AKA inflammatory pseudotumour, AKA inflammatory fibrosarcoma,[17] AKA plasma cell granuloma.[18][19]
Low-grade myofibroblastic sarcoma
General
- Rare ~ 100 cases in the literature.
- Usu. oral cavity or extremities.[20]
Microscopic
Features:
- Spindle cells in the storiform pattern[20] or in fasicles.
- Rare mitoses.
Images:
DDx:
- Atypical leiomyoma.
- GIST.
- Leiomyosarcoma.
IHC
Congenital-infantile fibrosarcoma
- Should not be confused with adult fibrosarcoma.
General
- Locally aggressive.
Microscopic
Features:[21]
- Spindle cell lesion.
Molecular
Characteristic translocation:[22]
- t(12;15)(p13;q25).
- Gene fusion ETV6-NTRK3.
- Same translocation in mesoblastic nephroma.
- Gene fusion ETV6-NTRK3.
Solitary fibrous tumour
Hemangiopericytoma
General
- Grouped with solitary fibrous tumour in the WHO classification; share same genetic NAB2-STAT6 fusion.[8][23]
- Thought to arise from the pericyte, a connective tissue cell of small vessels that is thought to be involved in flow regulation while others consider a fibroblastic nature.[24]
- Hematologic spread most common - to lungs.[25]
- Oncogenic osteomalacia - assoc. with hemangiopericytoma.[25]
- WHO grade II hemangiopericytoma (ICD-O: 9150/1), WHO grade III anaplastic hemangiopericytoma (ICD-O: 9150/3)
Presentation
- Usually painless mass, slow enlargement.
- May profusely bleed during resection.
- May invade bone.
Histology
- high cellular density.
- indistinct cell borders.
- random tumor cell orientation.
- little fibrosis.
- plenty reticulin.
- vascular with slit-like channels ("staghorn-like vessels").
IHC
- Vimentin +ve.
- CD34 +ve (often patchy, used to differentiate from SFT).
- STAT6 nuclear +ve.
- EMA +/-ve.
Radiology
- Intramedullary lytic mass.
- May be well-circumscribed.
- +/-Periosteal reaction.
- +/-Sclerotic border.
May be worked-up with angiography to distinguish from a vascular malformation.[26]
Location
- Usually extremities - femur or proximal tibial.[25]
Microscopic
Features:[26]
- Hypervascular lesion - key diagnostic feature.[27]
- Abundant thin-walled branching small vessels of variable size.
- May be described as "staghorn vessels" or "antler-like" vasculature.
- Cells may "onion-skin" around thin blood vessels.
- Abundant thin-walled branching small vessels of variable size.
- Spindle or ovoid shaped cells in nests or sheets.
DDx:
- Other vascular tumours.
- Vascular malformations.
- Synovial sarcoma.
- Dermatofibroma. (???)
IHC
- Vimentin +ve (usually).
- Desmin -ve (typical).
- Factor VIII -ve (marks endothelium).
- CD34 +ve.
- CD34 usu. -ve in synovial sarcoma.
- CD31 -ve (marks benign endothelium).
- vWF (von Willebrand factor) -ve.
May be in the DDx for meningioma:[28]
- EMA -ve.
- S100 -ve.
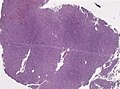
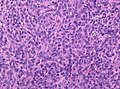
Images
Anaplastic hemangiopericytoma, STAT6 immunostaining. (WC/jensflorian)
Malignant
Low-grade fibromyxoid sarcoma
- AKA hyalinizing spindle cell tumour.
- Should not be confused with myxofibrosarcoma.
- Abbreviated LGFMS.
Adult fibrosarcoma
- AKA fibrosarcoma.
- Should not be confused with infantile fibrosarcoma.
General
- Malignant.
- Older adults.
- Locations: head & neck, extremities.
Microscopic
Feature:[29]
- Spindle cell lesion.
- Herring bone pattern - key feature.
- Mitoses.
DDx (herring bone):
- MPNST.
- Synovial sarcoma.
- Fibrosarcoma.
DDx:
- Dermatofibrosarcoma protuberans (DFSP) - t(17;22) COLA1/PDGFB.
- Congenital-infantile fibrosarcoma - t(12;15) ETV6/NTRK3.
- Solitary fibrous tumour.
- Synovial sarcoma - t(X;18) SYT/SSX.
- MPNST.
Images:
IHC
Features:[29]
- Vimentin.
- SMA.
Myxofibrosarcoma
- Should not be confused with low-grade fibromyxoid sarcoma.[30]
- AKA myxoid malignant fibrous histiocytoma or myxoid MFH.[31]
See also
References
- ↑ 1.0 1.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 601. ISBN 978-0781765275.
- ↑ 2.0 2.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 602. ISBN 978-0781765275.
- ↑ 3.0 3.1 Chung, EB.; Enzinger, FM. (Oct 1975). "Proliferative fasciitis.". Cancer 36 (4): 1450-8. PMID 1058047.
- ↑ Meis, JM.; Enzinger, FM. (Apr 1992). "Proliferative fasciitis and myositis of childhood.". Am J Surg Pathol 16 (4): 364-72. PMID 1566969.
- ↑ Gleason, BC.; Hornick, JL. (Apr 2008). "Inflammatory myofibroblastic tumours: where are we now?". J Clin Pathol 61 (4): 428-37. doi:10.1136/jcp.2007.049387. PMID 17938159.
- ↑ 6.0 6.1 6.2 6.3 el-Jabbour, JN.; Bennett, MH.; Burke, MM.; Lessells, A.; O'Halloran, A. (Jul 1991). "Proliferative myositis. An immunohistochemical and ultrastructural study.". Am J Surg Pathol 15 (7): 654-9. PMID 2058761.
- ↑ Lundgren, L.; Kindblom, LG.; Willems, J.; Falkmer, U.; Angervall, L. (May 1992). "Proliferative myositis and fasciitis. A light and electron microscopic, cytologic, DNA-cytometric and immunohistochemical study.". APMIS 100 (5): 437-48. PMID 1586481.
- ↑ 8.0 8.1 8.2 8.3 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 609. ISBN 978-0781765275.
- ↑ Watanabe, H.; Ishida, Y.; Nagashima, K.; Makino, T.; Norisugi, O.; Shimizu, T. (Feb 2008). "Desmoplastic fibroblastoma (collagenous fibroma).". J Dermatol 35 (2): 93-7. doi:10.1111/j.1346-8138.2008.00421.x. PMID 18271804.
- ↑ 10.0 10.1 10.2 Walker, KR.; Bui-Mansfield, LT.; Gering, SA.; Ranlett, RD. (Dec 2004). "Collagenous fibroma (desmoplastic fibroblastoma) of the shoulder.". AJR Am J Roentgenol 183 (6): 1766. PMID 15547225.
- ↑ 11.0 11.1 11.2 Miettinen, M.; Fetsch, JF. (Jul 1998). "Collagenous fibroma (desmoplastic fibroblastoma): a clinicopathologic analysis of 63 cases of a distinctive soft tissue lesion with stellate-shaped fibroblasts.". Hum Pathol 29 (7): 676-82. PMID 9670823.
- ↑ Mills, Stacey E; Carter, Darryl; Greenson, Joel K; Oberman, Harold A; Reuter, Victor E (2004). Sternberg's Diagnostic Surgical Pathology (4th ed.). Lippincott Williams & Wilkins. pp. 161. ISBN 978-0781740517.
- ↑ Takahara, M.; Ichikawa, R.; Oda, Y.; Uchi, H.; Takeuchi, S.; Moroi, Y.; Kiryu, H.; Furue, M. (Oct 2008). "Desmoplastic fibroblastoma: a case presenting as a protruding nodule in the dermis.". J Cutan Pathol 35 Suppl 1: 70-3. doi:10.1111/j.1600-0560.2007.00964.x. PMID 18544056.
- ↑ Macchia, G.; Trombetta, D.; Möller, E.; Mertens, F.; Storlazzi, CT.; Debiec-Rychter, M.; Sciot, R.; Nord, KH. (May 2012). "FOSL1 as a candidate target gene for 11q12 rearrangements in desmoplastic fibroblastoma.". Lab Invest 92 (5): 735-43. doi:10.1038/labinvest.2012.46. PMID 22411068.
- ↑ Attila T, Chen D, Gardiner GW, Ptak TW, Marcon NE (July 2006). "Gastric calcifying fibrous tumor". Can. J. Gastroenterol. 20 (7): 487–9. PMC 2659917. PMID 16858502. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2659917/.
- ↑ 16.0 16.1 16.2 16.3 16.4 Flucke, U.; van Krieken, JH.; Mentzel, T. (Jan 2011). "Cellular angiofibroma: analysis of 25 cases emphasizing its relationship to spindle cell lipoma and mammary-type myofibroblastoma.". Mod Pathol 24 (1): 82-9. doi:10.1038/modpathol.2010.170. PMID 20852591.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 610. ISBN 978-0781765275.
- ↑ URL: http://www.uptodate.com/contents/inflammatory-myofibroblastic-tumor-plasma-cell-granuloma-of-the-lung. Accessed on: 27 November 2011.
- ↑ Manohar, B.; Bhuvaneshwari, S. (Jan 2011). "Plasma cell granuloma of gingiva.". J Indian Soc Periodontol 15 (1): 64-6. doi:10.4103/0972-124X.82275. PMID 21772725.
- ↑ 20.0 20.1 20.2 20.3 Miyazawa, M.; Naritaka, Y.; Miyaki, A.; Asaka, S.; Isohata, N.; Yamaguchi, K.; Murayama, M.; Shimakawa, T. et al. (Sep 2011). "A low-grade myofibroblastic sarcoma in the abdominal cavity.". Anticancer Res 31 (9): 2989-94.
- ↑ Corsi, A.; Boldrini, R.; Bosman, C. (Oct 1994). "Congenital-infantile fibrosarcoma: study of two cases and review of the literature.". Tumori 80 (5): 392-400. PMID 7839472.
- ↑ Sheng, WQ.; Hisaoka, M.; Okamoto, S.; Tanaka, A.; Meis-Kindblom, JM.; Kindblom, LG.; Ishida, T.; Nojima, T. et al. (Mar 2001). "Congenital-infantile fibrosarcoma. A clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin-embedded tissues.". Am J Clin Pathol 115 (3): 348-55. doi:10.1309/3H24-E7T7-V37G-AKKQ. PMID 11242790.
- ↑ Schweizer, L.; Koelsche, C.; Sahm, F.; Piro, RM.; Capper, D.; Reuss, DE.; Pusch, S.; Habel, A. et al. (May 2013). "Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein.". Acta Neuropathol 125 (5): 651-8. doi:10.1007/s00401-013-1117-6. PMID 23575898.
- ↑ Gengler, C.; Guillou, L. (Jan 2006). "Solitary fibrous tumour and haemangiopericytoma: evolution of a concept.". Histopathology 48 (1): 63-74. doi:10.1111/j.1365-2559.2005.02290.x. PMID 16359538.
- ↑ 25.0 25.1 25.2 URL: http://emedicine.medscape.com/article/1255879-overview. Accessed on: 2 May 2010.
- ↑ 26.0 26.1 URL: http://emedicine.medscape.com/article/1255879-diagnosis. Accessed on: 2 May 2010.
- ↑ 27.0 27.1 Enzinger & Weiss's Soft Tissue Tumors. 4th Ed. PP.1007-13. ISBN 0-323-01200-0.
- ↑ Croul, SE. 8 November 2010.
- ↑ 29.0 29.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 611. ISBN 978-0781765275.
- ↑ Mentzel, T.; Katenkamp, D.; Fletcher, CD. (Mar 1996). "[Low malignancy myxofibrosarcoma versus low malignancy fibromyxoid sarcoma. Distinct entities with similar names but different clinical course].". Pathologe 17 (2): 116-21. PMID 8650138.
- ↑ Fujimura, T.; Okuyama, R.; Terui, T.; Okuno, K.; Masu, A.; Masu, T.; Chiba, S.; Kunii, T. et al. (Aug 2005). "Myxofibrosarcoma (myxoid malignant fibrous histiocytoma) showing cutaneous presentation: report of two cases.". J Cutan Pathol 32 (7): 512-5. doi:10.1111/j.0303-6987.2005.00368.x. PMID 16008697.