Esophagus
Esophagus connects the pharynx to the stomach. It is afflicted by tumours on occasion. Probably the most common affliction is gastroesophageal reflux disease (GERD). Most biopsies revolve around the questions: 1. intestinal metaplasia? 2. dysplasia? and 3. cancer?
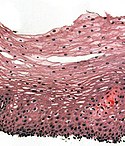
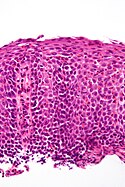
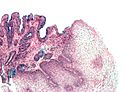
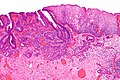
Normal esophagus
General:
- Stratified squamous non-keratinized epithelium.
Normal (esophageal) squamous epithelium:
- Should "mature" to the surface like good stratified squamous epithelium does.
- No nuclei at luminal surface.
- Cells should become less hyperchromatic as you go toward the lumen.
- Mitoses should be rare and should NOT be above the basal layer.
- Inflammatory cells should be very rare.
Sign out
ESOPHAGUS, DISTAL, BIOPSY: - COLUMNAR EPITHELIUM WITH MODERATE CHRONIC INFLAMMATION. - REACTIVE SQUAMOUS EPITHELIUM. - NEGATIVE FOR INTESTINAL METAPLASIA. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY.
Diagnoses
Common
- Normal.
- Metaplasia (Barrett's esophagus).
- Dysplasia.
- Adenocarcinoma.
Less common
- Squamous cell carcinoma.
- Eosinophilic esophagitis.
- Candidiasis.
- CMV esophagitis.
Tabular summary
Simplified overview
| Entity | Key feature | Other features | IHC/Special | Clinical | Image |
| Normal | squamous epi. matures to surface | no inflammation, no atypia | - | - | |
| GERD | inflammation (eosinophils, lymphocytes) | elongated (epithelial) papillae, basal cell hyperplasia | incr. risk of Barrett's | ||
| Eosinophilic esophagitis | abundant eosinophils | elongated (epithelial) papillae, basal cell hyperplasia, lymphocytes | unresponsive to PPIs | ||
| Barrett's type change | goblet cells | no dysplasia | Alcian blue +ve | incr. risk of adenocarcinoma | |
| Dysplasia, low grade | nuclear crowding at surface | hyperchromasia, mild arch. complexity, no necrosis | incr. risk of carcinoma | ||
| Dysplasia, high grade | cribriforming and/or necrosis | nuclei often round & large, hyperchromasia | marked incr. risk of carcinoma |
Columnar dysplasia
| Entity | Surface maturation | Architecture | Cytology | Other | Clinical | Image |
| Normal | matures | round glands | no nuclear atypia | - | - | Image |
| Barrett's esophagus | matures | round glands, normal gland density | +/-scant nuclear atypia | goblet cells | clinical diagnosis | Image |
| Indefinite for columnar dysplasia | minimal maturation or cannot see surface | round glands, normal gland density | mild nuclear atypia, nuclear pseudostratification, no necrosis | - | follow-up | Image |
| Low-grade columnar dysplasia | minimal-to-scant maturation | round glands, +/-rare budding, increased gland density | mild-to-moderate nuclear atypia, nuclear pseudostratification, no necrosis | - | follow-up | Image |
| High-grade columnar dysplasia | no maturation | incr. density of irregular glands with budding and/or rare cribriforming and/or gland dilation | moderate-to-marked nuclear atypia (usu. plump round nuclei), hyperchromasia, +/-necrosis | - | EMR, surgery | Image |
| Intramucosal adenocarcinoma | no maturation | single cells or back-to-back irregular glands with budding and/or cribriforming and/or gland dilation or glands with long axis along muscularis mucosae | moderate-to-marked nuclear atypia - usu. round large nuclei, hyperchromasia, +/-necrosis | - | EMR, surgery | Image |
Columnar dysplasia - another table
| Feature | Indefinite for columnar dysplasia | Low-grade columnar dysplasia | High-grade columnar dysplasia | Intramucosal carcinoma (IMCa) | Utility |
|---|---|---|---|---|---|
| Depth of glands | superficial only | superficial only | superficial/deep | deep | low vs. high |
| Gland density | normal | near normal | increased | back-to-back | low vs. high vs. IMCa |
| Gland morphology | round | round | irregular/rare cribriforming | irregular/cribriform/sheeting | low vs. high vs. IMCa |
| Necrosis | none | none | may be present | may be present | low vs. high & IMCa |
| Hyperchromasia | +/- | present | present | present | indef. vs. low |
| Palisaded/crowded nuclei | present | present | absent/present | uncommon | low vs. high |
| Round nuclei + enlargement | absent | absent | present/absent | present | low vs. high |
| Desmoplasia | absent | absent | absent | +/- (uncommon) | high vs. IMCa |
| Surface involvement | present (required) | present (required) | +/- | +/- | low vs. high |
Indications
- Pyrosis = heartburn.[1]
Infectious esophagitis
Is a relatively common problem, especially in those that live at the margins (EtOH abusers) and immunosuppressed individuals (HIV/AIDS).
Useful stains
- PAS.
- Gram stain.
Overview
Candida esophagitis
- AKA esophageal candidiasis.
Gross (endoscopic)
Features:
- White patches.
DDx (endoscopic):[2]
Microscopic
Features:
- Worm-like micro-organisms - key feature.
- Pseudohyphae (single cells).
- Thickness ~ 1/3-1/2 of squamous cell nucleus.
- Should be within (squamous) epithelium.
- Superficial inflammation - esp. neutrophils - important.
Notes:
- On top of epithelium does not count,[3] i.e. it is likely an artifact.
- Bacilli and cocci may accompany the candida. They are typically ignored.
DDx:
- Acute esophagitis - no candida seen.
Image
Sign out
ESOPHAGUS, BIOPSY: - ESOPHAGITIS WITH FUNGAL ORGANISMS CONSISTENT WITH CANDIDA.
ESOPHAGUS, BIOPSY: - ACUTE ESOPHAGITIS WITH FUNGAL ORGANISMS CONSISTENT WITH CANDIDA. - NEGATIVE FOR INTESTINAL METAPLASIA. - NEGATIVE FOR DYSPLASIA.
Cytomegalovirus esophagitis
Microscopic
Features:
- Classically at the base of the ulcer; within endothelial cells - key point.
Note:
- Biopsying the the base of an ulcer usually just yields (non-diagnostic) necrotic debris; so, clinicians are told to biopsy the edge of the lesion. A suspected CMV infection is the exception to this rule!
Herpes esophagitis
General
Etiology:
Gross/endoscopic
Features:
- Ulcers with a "punched-out" appearance with a brown/red edge.
Images
www:
Microscopic
Features (3 Ms):
- Moulding.
- Multinucleation.
- Margination of chromatin.
Images
Human papillomavirus esophagitis
General:
Microscopic
Features:
- Koilocytes:
- Perinuclear clearing.
- Nuclear changes.
- Size similar (or larger) to those in the basal layer of the epithelium.
- Nuclear enlargement should be evident on low power, i.e. 25x. [7]
- Central location - nucleus should be smack in the middle of the cell.
Images:
Other
The group of conditions doesn't fit neatly with the others. It is a mixture of different non-neoplastic conditions.
Gastroesophageal reflux disease
- Abbreviated GERD or GORD (gastro-oesophageal reflux disease).
General
Clinical:
- Usually chest pain
- +/-Abdominal pain.
- +/-Vomiting.
- +/-Blood loss.
Treatment:
- Treated with proton pump inhibitors (PPIs).
DDx (clinical):
Gross
- Erythema.
- Erosions.
- +/-Ulceration.
Note:
- Many be graded using Savary-Miller classification.
Images:
Microscopic
Features:
- Basal cell hyperplasia;[4] > 3 cells thick or >15% of epithelial thickness.
- Papillae elongated; papillae reach into the top 1/3 of the epithelial layer.[5]
- Inflammation, esp. eosinophils, lymphocytes with convoluted nuclei ("squiggle cells").
- +/-Intraepithelial edema.
- +/-Apoptotic cells.[6]
Notes:
- Intraepithelial cells with irregular nuclear contours, "squiggle cells" (T lymphocytes[7]), may mimic neutrophils.
- Changes may be focal.
- PPI effect may be seen in a concurrent gastric biopsy.
DDx:
- Eosinophilic esophagitis - characterized by similar histomorphologic features. The key difference is: more eosinophils.
- Barrett's esophagus - intestinal metaplasia may be minimal.
Images:
Sign out
Poorly oriented
ESOPHAGUS, BIOPSY: - SQUAMOUS MUCOSA WITH BASAL CELL HYPERPLASIA, INTRAEPITHELIAL EDEMA AND RARE INTRAEPITHELIAL EOSINOPHILS -- COMPATIBLE WITH GASTROESOPHAGEAL REFLUX.
Columnar epithelium present
ESOPHAGUS, BIOPSY: - SQUAMOUS MUCOSA WITH BASAL CELL HYPERPLASIA, INTRAEPITHELIAL EDEMA AND RARE INTRAEPITHELIAL EOSINOPHILS -- COMPATIBLE WITH GASTROESOPHAGEAL REFLUX. - COLUMNAR EPITHELIUM WITH MODERATE CHRONIC INACTIVE INFLAMMATION. - NEGATIVE FOR INTESTINAL METAPLASIA. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY.
Ulceration present
ESOPHAGUS, DISTAL, BIOPSY: - SQUAMOUS MUCOSA WITH BASAL CELL HYPERPLASIA, INTRAEPITHELIAL EDEMA, RARE INTRAEPITHELIAL EOSINOPHILS AND EVIDENCE OF ULCERATION -- COMPATIBLE WITH GASTROESOPHAGEAL REFLUX. - COLUMNAR EPITHELIUM WITH MODERATE CHRONIC INACTIVE INFLAMMATION. - NEGATIVE FOR INTESTINAL METAPLASIA. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY. COMMENT: PAS-D staining is negative for microorganisms.
BE and GERD present
ESOPHAGUS (DISTAL), BIOPSY: - COLUMNAR EPITHELIUM WITH INTESTINAL METAPLASIA AND MODERATE CHRONIC INFLAMMATION, SEE COMMENT. - SQUAMOUS MUCOSA WITH BASAL CELL HYPERPLASIA, INTRAEPITHELIAL EDEMA AND RARE INTRAEPITHELIAL EOSINOPHILS -- COMPATIBLE WITH GASTROESOPHAGEAL REFLUX. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY. COMMENT: The findings are consistent with Barrett's esophagus in the appropriate endoscopic setting.
Eosinophilic esophagitis
- Abbreviated EE.
Erosive esophagitis
DDx
- Infections.
- Crohn's disease.
- Pill esophagitis.
Work-up
Pill esophagitis
Classic causes:
- Alendronate (Fosamax) - for osteoporosis.
- Iron - can be demonstrated with Prussian blue stain.
- Doxycycline.
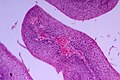
Esophageal varices
General
- Arise due to portal hypertension.
- This is usually due to cirrhosis that in turn is most often due to alcoholism.
- Usually a clinical diagnosis.
- Major cause of death in cirrhotics.[9]
Gross
- Prominent blood vessels in the distal eosphagus.
Note:
Image:
Microscopic
Features:
- Large dilated submucosal veins - key feature.
- +/-Blood.
Image:
Acute esophagitis
Benign esophageal stricture
Preneoplastic
Barrett esophagus
Neoplastic
Columnar dysplasia of the esophagus
- AKA esophageal columnar dysplasia, abbreviated ECD.[12]
- AKA dysplasia in the columnar-lined esophagus.[13]
- AKA columnar epithelial dysplasia.[14]
General
- Arises in the setting of Barrett esophagus.
Classification
- Indefinite for dysplasia.
- Low grade dysplasia.
- High grade dysplasia.
Management
Low grade dysplasia & indefinite for dysplasia:
- Follow-up.
High grade dysplasia:
- Endoscopic mucosal resection.[15]
- Surgical resection (esophagectomy).
Microscopic
Features to assess:[16]
- Lack of surface maturation - very common, occasionally absent.[17]
- Lack of lighter staining at surface.
- Nuclear crowding at surface.
- Nuclei at the surface not smaller.
- Architecture - esp. at low power.
- Glands not round.
- Low-grade feature: gland budding.
- High-grade features: cribriforming, cystic dilation, necrotic debris.
- Gland density:
- Increased & round - think low-grade dysplasia.
- Increased & irregular - think high-grade dysplasia.
- Glands not round.
- Cytology, esp. at high magnification.
- Nuclear abnormalities in: size, staining, shape.
- Loss of "nuclear polarity" = high-grade feature
- Loss of palisaded appearance, rounding-up of nuclei.
- Inflammation, erosions & ulceration.
- Marked inflammation should prompt consideration of knocking down the diagnosis one step, i.e. low-grade becomes indefinite or high-grade becomes low-grade.
Negatives:
- No desmoplasia.
- Stromal fibrotic reaction to the tumour.
- Desmoplasia is rare in the superficial esophagus.[18]
- Stromal fibrotic reaction to the tumour.
- No single cells.
- No extensive back-to-back glands.
Notes:
- Changes similar to those see in colorectal tubular adenomas; however, what would be low-grade dysplasia in the rectum is high-grade dysplasia in the esophagus.
- Presence of goblet cells suggests it is not dysplasia.[19]
- Desmoplasia present = invasive adenocarcinoma.[20]
- Some literature suggests community pathologists should not make this call, i.e. it should be diagnosed by an expert.[21]
DDx:
Images
Indefinite for columnar dysplasia:
Low-grade columnar dysplasia:
High-grade columnar dysplasia:
Sign out
ESOPHAGUS, DISTAL, BIOPSY: - LOW-GRADE COLUMNAR EPITHELIAL DYSPLASIA, SEE COMMENT. - COLUMNAR EPITHELIUM WITH GOBLET CELL METAPLASIA. - REACTIVE SQUAMOUS EPITHELIUM. COMMENT: This was reviewed with Dr. X and they agree with the diagnosis.
Alternate
ESOPHAGUS, 30 CM, BIOPSY: - LOW-GRADE COLUMNAR DYSPLASIA WITH INTESTINAL METAPLASIA AND MILD CHRONIC INFLAMMATION. - NEGATIVE FOR MALIGNANCY.
Squamous dysplasia of the esophagus
- AKA eosphageal squamous dysplasia.
General
- Precursor of esophageal squamous cell carcinoma.[24]
- Common in China.[24]
- Not very common in North America.
Microscopic
Features:
- Squamous cell nuclear atypia.
- Lack of maturation to the surface.
Note:
- Grading differences between Western pathologists and those of the east.[24]
DDx:
- Reactive changes.
- Esophageal squamous cell carcinoma.
Images
A set of cases from Japan:[25]
- Mild squamous dysplasia (nih.gov).
- Moderate squamous dysplasia (nih.gov).
- Severe squamous dysplasia (nih.gov).
- Carcinoma in situ (nih.gov).
- Squamous cell carcinoma of the esophagus (nih.gov).
IHC
- Ki-67 may be useful:[26]
- Reactive changes/normal: ~98% negative, ~2% intermediate.
- Low-grade esophageal squamous intraepithelial neoplasia (LGESIN): ~80% intermediate, ~20% negative.
- High-grade esophageal squamous intraepithelial neoplasia (HGESIN): ~37% intermediate, ~63% strong.
Definitions:[26]
- Negative defined as: < 25% of epithelium +ve and staining only in lower quarter of epithelium.
- Intermediate defined: >=25% and <=50% of epithelium +ve and only in the lower half of the epithelium.
- Strong defined: >50% of epithelium +ve or upper half of epithelium.
Leiomyoma of the esophagus
General
- Benign.
- Uncommon.
- Before the time of GISTs - this was a relatively common diagnosis.
- Like leiomyomas elswhere.
Microscopic
- See: Leiomyoma.
DDx:
Gastrointestinal stromal tumour
Cancer
General
- Proximal esophagus: squamous cell carcinoma.
- Distal esophagus: adenocarcinoma arising from Barrett's esophagus.
Risks:
Squamous cell carcinoma of the esophagus
- AKA esophageal squamous cell carcinoma, abbreviated esophageal SCC.
General
- Like squamous cell carcinoma elsewhere.
Risk factors:[27]
- Alcohol consumption.
- Tobacco use.
- Food with nitrosamines.
- Burning-hot beverages.
Note:
- Reflux is not a risk factor for esophageal SCC.
Microscopic
- See Squamous carcinoma.
Note:
- Just to make things confusing, the Staging of early SCC differs from that of early adenocarcinoma!
DDx:
Images
Sign out
ESOPHGAUS, BIOPSY: - INVASIVE SQUAMOUS CELL CARCINOMA, KERATINIZING, MODERATELY DIFFERENTIATED. COMMENT: Benign squamous epithelium at least partially overlies the invasive squamous cell carcinoma; this may mask the true extent of the lesion on endoscopy.
Micro
The sections show a squamous mucosa with focal moderate atypia of the squamous cells, keratinization and easily identified mitotic figures. The atypical cells are partially covered by benign squamous cells, and there is a very sharp transition between the cells with atypia and those without. The atypical squamous cells extend into the subepithelial tissue in irregularly shaped nests and cords. A small amount of benign muscle is present.
Esophageal adenocarcinoma
- AKA adenocarcinoma of the esophagus.
General
- Often a prognosis poor - as diagnosed in a late stage.
- May be difficult to distinguish from adenocarcinoma of the stomach.
- By convention (in the CAP checklist) gastroesophageal junction carcinomas are staged as esophageal carcinomas.[28]
Tx
- Adenocarcinoma in situ (AIS) - may be treated with endoscopic mucosal resection & follow-up.[15]
- Surgery - esophagectomy.
Esophagus vs. stomach
The convention is it's esophageal if both of the following are true:[29]
- Epicenter of tumour is in the esophagus.
- Barrett's mucosa is present.
Microscopic
Features:
- Adenocarcinoma:
- Cell clusters that form glands.
- Nuclear atypia of malignancy:
- Size variation.
- Shape variation.
- Staining variation.
- Mitoses common.
Images
Grading
Graded like other adenocarcinoma:[29]
- >95 % of tumour in glandular arrangement = well-differentiated.
- 95-50% of tumour in glandular arrangement= moderately-differentiated.
- <50% of tumour in glandular arrangment = poorly-differentiated.
Staging
Early esophageal adenocarcinoma has its own staging system:[30][31]
- M1 = lamina propria.
- M2 = superficial muscularis mucosae.
- M3 = submucosa.
- M4 = muscularis propria.
IHC
- CK7 +ve.
- CK20 +ve.
To rule-out SCC:
- p63 -ve.
- HWMK -ve.
Weird stuff
- Inflammatory polyp - assoc. trauma/previous intervention.
- Giant fibrovascular polyp - loose connective tissue covered with squamous epithelium.
- Granular cell tumour.
- Squamous papilloma - koilocytes.
- Heterotopic gastric mucosa ("inlet patch") - benign appearing gastric mucosa.
Granular cell tumour
Microscopic
Features:
- Abundant eosinophilic granular cytoplasm key feature.
- Granules:
- Size: 1-3 micrometers.
- Poorly demarcated.
- Granules:
- Usu. bland (cytologically non-malignant) nuclei.
Images
Esophagitis dissecans superficials
General
- Rare & benign condition that resolves without lasting pathology.[32]
- Case report - chronic with strictures.[33]
- Sloughing of large fragments of the esophageal mucosa - seen on endoscopy.
Microscopic
Features:[32]
- Flaking of superficial squamous epithelium.
- Focal bullous separation of the layers.
- Parakeratosis.
- Variable acute or chronic inflammation.
Glycogenic acanthosis of the esophagus
General
- Uncommon.
- Benign.
- Possible association with ingestion of hot liquids.[34]
Gross/endoscopic
- Distinctive endoscopic appearance - grey/white raised lesion.[34]
Image:
Microscopic
Features:[34]
- Squamous epithelium with:
- Superficial clearing of the cytoplasm.
- Thickening.
Images:
Achalasia
General
- Uncommon.
- Risk factor for squamous cell carcinoma (in men and women) and adenocarcinoma (in men).[35]
- Due to loss of ganglion cells.[36]
Clinical:
- Dysphagia (difficulty swallowing) liquids and solids.[36]
Microscopic
Features:[37]
- Mucosa typically normal - even in long-standing achalasia.
Note:[37]
- Achalasia seen in the context of a resection usually has inflammation.
- Post-Heller myotomy often has inflammation.
Sign out
ESOPHAGUS, BIOPSY: - SQUAMOUS EPITHELIUM WITH A MILD DEEP LYMPHOCYTIC INFILTRATE, EDEMA, AND REACTIVE CHANGES, NO EOSINOPHILS APPARENT. - SCANT COLUMNAR EPITHELIUM WITH MINIMAL STROMA, NO APPARENT SIGNIFICANT PATHOLOGY. - NEGATIVE FOR INTESTINAL METAPLASIA. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY.
Alternate
GASTROESOPHAGEAL JUNCTION, BIOPSY: - COLUMNAR EPITHELIUM WITH MODERATE CHRONIC INFLAMMATION. - REACTIVE SQUAMOUS EPITHELIUM. - NEGATIVE FOR INTESTINAL METAPLASIA. - NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY.
Esophageal inlet patch
General
- Benign and likely not of any significance.[38]
Gross
- Proximal esophagus - salmon coloured lesion.[38]
Microscopic
Features:
- Gastric mucosa.[39]
Image:
Squamous papilloma of the eosphagus
- AKA esophageal squamous papilloma.
General
- Uncommon.
Microscopic
Features:
- Papillomaous projections - low power.
Image
www:
See also
References
- ↑ URL: http://dictionary.reference.com/browse/pyrosis. Accessed on: 21 June 2010.
- ↑ Odze, Robert D.; Goldblum, John R. (2009). Surgical pathology of the GI tract, liver, biliary tract and pancreas (2nd ed.). Saunders. pp. 244. ISBN 978-1416040590.
- ↑ ALS. 4 October 2010.
- ↑ Steiner, SJ.; Kernek, KM.; Fitzgerald, JF. (May 2006). "Severity of basal cell hyperplasia differs in reflux versus eosinophilic esophagitis.". J Pediatr Gastroenterol Nutr 42 (5): 506-9. doi:10.1097/01.mpg.0000221906.06899.1b. PMID 16707971.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 804. ISBN 0-7216-0187-1.
- ↑ Wetscher GJ, Schwelberger H, Unger A, et al. (December 1998). "Reflux-induced apoptosis of the esophageal mucosa is inhibited in Barrett's epithelium". Am. J. Surg. 176 (6): 569–73. PMID 9926792.
- ↑ Cucchiara, S.; D'Armiento, F.; Alfieri, E.; Insabato, L.; Minella, R.; De Magistris, TM.; Scoppa, A. (Nov 1995). "Intraepithelial cells with irregular nuclear contours as a marker of esophagitis in children with gastroesophageal reflux disease.". Dig Dis Sci 40 (11): 2305-11. PMID 7587806.
- ↑ Genevay, M.; Rubbia-Brandt, L.; Rougemont, AL. (Jun 2010). "Do eosinophil numbers differentiate eosinophilic esophagitis from gastroesophageal reflux disease?". Arch Pathol Lab Med 134 (6): 815-25. doi:10.1043/1543-2165-134.6.815. PMID 20524860.
- ↑ Tsochatzis, EA.; Triantos, CK.; Garcovich, M.; Burroughs, AK. (Feb 2011). "Primary prevention of variceal hemorrhage.". Curr Gastroenterol Rep 13 (1): 3-9. doi:10.1007/s11894-010-0160-x. PMID 21086193.
- ↑ Burton, Julian L.; Rutty, Guy N. (2010). The Hospital Autopsy A Manual of Fundamental Autopsy Practice (3rd ed.). Oxford University Press. pp. 140. ISBN 978-0340965146.
- ↑ URL: http://www.pathguy.com/lectures/guts.htm. Accessed on: 24 April 2013.
- ↑ Feng, W.; Zhou, Z.; Peters, JH.; Khoury, T.; Zhai, Q.; Wei, Q.; Truong, CD.; Song, SW. et al. (Aug 2011). "Expression of insulin-like growth factor II mRNA-binding protein 3 in human esophageal adenocarcinoma and its precursor lesions.". Arch Pathol Lab Med 135 (8): 1024-31. doi:10.5858/2009-0617-OAR2. PMID 21809994.
- ↑ Levine, DS. (Sep 1997). "Management of dysplasia in the columnar-lined esophagus.". Gastroenterol Clin North Am 26 (3): 613-34. PMID 9309409.
- ↑ Hamilton, SR.; Smith, RR. (Mar 1987). "The relationship between columnar epithelial dysplasia and invasive adenocarcinoma arising in Barrett's esophagus.". Am J Clin Pathol 87 (3): 301-12. PMID 3825997.
- ↑ 15.0 15.1 Sampliner RE (March 2009). "Endoscopic Therapy for Barrett's Esophagus". Clin. Gastroenterol. Hepatol.. doi:10.1016/j.cgh.2009.03.011. PMID 19306943.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 46. ISBN 978-0443066573.
- ↑ Lomo, LC.; Blount, PL.; Sanchez, CA.; Li, X.; Galipeau, PC.; Cowan, DS.; Ayub, K.; Rabinovitch, PS. et al. (Apr 2006). "Crypt dysplasia with surface maturation: a clinical, pathologic, and molecular study of a Barrett's esophagus cohort.". Am J Surg Pathol 30 (4): 423-35. PMID 16625087.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 49. ISBN 978-0443066573.
- ↑ GAG. January 2009.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 54. ISBN 978-0443066573.
- ↑ Alikhan, M.; Rex, D.; Khan, A.; Rahmani, E.; Cummings, O.; Ulbright, TM. (Jul 1999). "Variable pathologic interpretation of columnar lined esophagus by general pathologists in community practice.". Gastrointest Endosc 50 (1): 23-6. PMID 10385717.
- ↑ 22.0 22.1 Odze, RD. (Aug 2009). "Barrett esophagus: histology and pathology for the clinician.". Nat Rev Gastroenterol Hepatol 6 (8): 478-90. doi:10.1038/nrgastro.2009.103. PMID 19581906.
- ↑ Riddell, RH.; Odze, RD. (Oct 2009). "Definition of Barrett's esophagus: time for a rethink--is intestinal metaplasia dead?". Am J Gastroenterol 104 (10): 2588-94. doi:10.1038/ajg.2009.390. PMID 19623166.
- ↑ 24.0 24.1 24.2 Dry, SM.; Lewin, KJ. (Feb 2002). "Esophageal squamous dysplasia.". Semin Diagn Pathol 19 (1): 2-11. PMID 11936262.
- ↑ 25.0 25.1 25.2 25.3 Terada, T. (2013). "A clinicopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies.". Int J Clin Exp Pathol 6 (2): 191-8. PMID 23330004.
- ↑ 26.0 26.1 Wang, WC.; Wu, TT.; Chandan, VS.; Lohse, CM.; Zhang, L. (Oct 2011). "Ki-67 and ProExC are useful immunohistochemical markers in esophageal squamous intraepithelial neoplasia.". Hum Pathol 42 (10): 1430-7. doi:10.1016/j.humpath.2010.12.009. PMID 21420715.
- ↑ Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 104 Q1. ISBN 978-1416025887.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/Esophagus_11protocol.pdf. Accessed on: 6 April 2012.
- ↑ 29.0 29.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 168. ISBN 978-0781765275.
- ↑ Pech, O.; May, A.; Rabenstein, T.; Ell, C. (Nov 2007). "Endoscopic resection of early oesophageal cancer.". Gut 56 (11): 1625-34. doi:10.1136/gut.2006.112110. PMC 2095648. PMID 17938435. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2095648/.
- ↑ Thosani, N.; Singh, H.; Kapadia, A.; Ochi, N.; Lee, JH.; Ajani, J.; Swisher, SG.; Hofstetter, WL. et al. (Nov 2011). "Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis.". Gastrointest Endosc. doi:10.1016/j.gie.2011.09.016. PMID 22115605.
- ↑ 32.0 32.1 32.2 Carmack, SW.; Vemulapalli, R.; Spechler, SJ.; Genta, RM. (Dec 2009). "Esophagitis dissecans superficialis ("sloughing esophagitis"): a clinicopathologic study of 12 cases.". Am J Surg Pathol 33 (12): 1789-94. doi:10.1097/PAS.0b013e3181b7ce21. PMID 19809273.
- ↑ Coppola, D.; Lu, L.; Boyce, HW. (Oct 2000). "Chronic esophagitis dissecans presenting with esophageal strictures: a case report.". Hum Pathol 31 (10): 1313-7. doi:10.1053/hupa.2000.18470. PMID 11070124.
- ↑ 34.0 34.1 34.2 Lopes, S.; Figueiredo, P.; Amaro, P.; Freire, P.; Alves, S.; Cipriano, MA.; Gouveia, H.; Sofia, C. et al. (May 2010). "Glycogenic acanthosis of the esophagus: an unusually endoscopic appearance.". Rev Esp Enferm Dig 102 (5): 341-2. PMID 20524767.
- ↑ Zendehdel, K.; Nyrén, O.; Edberg, A.; Ye, W. (Jan 2011). "Risk of esophageal adenocarcinoma in achalasia patients, a retrospective cohort study in Sweden.". Am J Gastroenterol 106 (1): 57-61. doi:10.1038/ajg.2010.449. PMID 21212754.
- ↑ 36.0 36.1 Blatnik, JA.; Ponsky, JL. (Jan 2014). "Advances in the Treatment of Achalasia.". Curr Treat Options Gastroenterol. doi:10.1007/s11938-013-0007-2. PMID 24395614.
- ↑ 37.0 37.1 Kjellin, AP.; Ost, AE.; Pope, CE. (2005). "Histology of esophageal mucosa from patients with achalasia.". Dis Esophagus 18 (4): 257-61. doi:10.1111/j.1442-2050.2005.00478.x. PMID 16128783.
- ↑ 38.0 38.1 Chong, VH. (Jan 2013). "Clinical significance of heterotopic gastric mucosal patch of the proximal esophagus.". World J Gastroenterol 19 (3): 331-8. doi:10.3748/wjg.v19.i3.331. PMID 23372354.
- ↑ 39.0 39.1 Behrens, C.; Yen, PP. (2011). "Esophageal inlet patch.". Radiol Res Pract 2011: 460890. doi:10.1155/2011/460890. PMID 22091379.