Difference between revisions of "Duodenum"
| Line 80: | Line 80: | ||
**Too much blue and epithelium in the wrong place. | **Too much blue and epithelium in the wrong place. | ||
====More==== | ====More==== | ||
*H. pylori only in areas of [[Gastric heterotopia of the duodenum|gastric metaplasia]].<ref>El-Zimaity. 18 October 2010.</ref> | *[[Helicobacter duodenitis|H. pylori]] only in areas of [[Gastric heterotopia of the duodenum|gastric metaplasia]].<ref>El-Zimaity. 18 October 2010.</ref> | ||
===Duodenal nodules DDX=== | ===Duodenal nodules DDX=== | ||
Revision as of 18:50, 5 February 2024
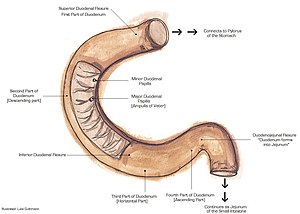
The duodenum is the first part of the small bowel and receives food from the stomach. It is accessible by EGD (esophagogastroduodenoscopy) and frequently biopsied.
An introduction to gastrointestinal pathology is in the gastrointestinal pathology article.
The clinical history is often: r/o celiac or r/o giardia.
Getting started
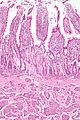
Normal duodenum
- Abbreviated ND.
General
- Very common.
Microscopic
- Three tall villi.
- Few intraepithelial lymphocytes; < 1 lymphocyte / 4 epithelial cells.
- No (pink) subepithelial collagen band.
- Predominant lamina propria cell: plasma cells.
- Lack of plasma cells suggests common variable immunodeficiency (CVID).[1]
- No organisms in lumen.
DDx:
- Intestinal metaplasia of the stomach - foveolar epithelium + other histologic components of the stomach.
- Chronic duodenitis - foveolar epithelium, Brunner's gland hyperplasia.
Sign out
Duodenum, Biopsy: - Small bowel mucosa and Brunner's glands within normal limits.
Duodenum, Biopsy: - Small bowel mucosa within normal limits.
Duodenum, Biopsy: - Small bowel mucosa within normal limits. - NEGATIVE for findings suggestive of celiac disease.
Small Bowel (Duodenum), Biopsy: - Small bowel mucosa within normal limits. - NEGATIVE for findings suggestive of celiac disease.
Block letters
DUODENUM, BIOPSY: - SMALL BOWEL MUCOSA AND BRUNNER'S GLANDS WITHIN NORMAL LIMITS.
DUODENUM, BIOPSY: - SMALL BOWEL MUCOSA WITHIN NORMAL LIMITS.
DUODENUM, BIOPSY: - SMALL BOWEL MUCOSA WITHIN NORMAL LIMITS. - NEGATIVE FOR FINDINGS SUGGESTIVE OF CELIAC DISEASE.
SMALL BOWEL (DUODENUM), BIOPSY: - SMALL BOWEL MUCOSA WITHIN NORMAL LIMITS. - NEGATIVE FOR FINDINGS SUGGESTIVE OF CELIAC DISEASE.
Basic DDx
- Celiac sprue.
- Intraepithelial lymphocytes - key feature.
- Loss of villi.
- Giardia.
- Like celiac... but giardia organisms.
- Adenomas.
- Too much blue - similar to colonic adenomas.
- Cancer.
- Too much blue and epithelium in the wrong place.
More
- H. pylori only in areas of gastric metaplasia.[2]
Duodenal nodules DDX
| Duodenal nodule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Benign (common) | Neoplastic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brunner's gland | Heterotopic gastric mucosa | Lymphoid nodule | Adenoma | NET | Paraganglioma | Prolapsed gastric polyp | Metastasis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Infections of the duodenum[3]
Common:
Rare:
- Cryptosporidia.
- Microsporidia.
- Isospora belli.
- Cyclospora.
- MAC (Mycobacterium avium complex).
- CMV (cytomegalovirus).
- Cryptococcus neoformans.
Common stuffs
Gastric heterotopia of the duodenum
- AKA heterotopic gastric mucosa.
Celiac sprue
- AKA celiac disease.
Giardiasis
Acute duodenitis
- Abbreviated AD.
Chronic duodenitis
General
- This is not very well defined as plasma cells are present in a normal duodenum.
Gross
- Duodenal erythema.
Microscopic
Features:
- "Abundant" lamina propria plasma cells.
- Villous blunting.
- Brunner's gland hyperplasia.
DDx:
Sign out
DUODENUM, BIOPSY: - MODERATE NON-SPECIFIC CHRONIC DUODENTIS (SMALL BOWEL MUCOSA WITH VILLOUS BLUNTING, PROMINENT BRUNNER'S GLANDS, ABUNDANT LAMINA PROPRIA PLASMA CELLS AND OCCASIONAL INTRAEPITHELIAL LYMPHOCYTES, WITHOUT FOVEOLAR METAPLASIA). - NEGATIVE FOR DYSPLASIA.
Peptic duodenitis
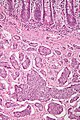
Brunner's gland hyperplasia
- Brunner's gland hamartoma redirects here.
General
- Benign.
- Usually asymptomatic.[5]
Note:
- The AFIP uses the term Brunner's gland hamartoma for lesions > 5 mm.[6]
- Multiple lesions less than 5 mm are hyperplasia.
Gross
- Nodularity of the duodenum.
Microscopic
Features:
- Prominent Brunner's gland.
- Tubular structures - formed by cells abundant cytoplasm that is clear with eosinophilic "cobwebs" and a round, small basal nucleus without a nucleolus.
- Brunner's glands close to the surface epithelium - key feature.[7]
- +/-Pancreatic acini and ducts.[6]
DDx:
- Foveolar metaplasia (isolated) - see peptic duodenitis.
- Peptic duodenitis.
Image:
Sign out
DUODENUM, BIOPSY: - CONSISTENT WITH BRUNNER'S GLAND HYPERPLASIA. - SMALL BOWEL MUCOSA WITHOUT SIGNIFICANT PATHOLOGY.
DUODENUM, BIOPSY: - SMALL BOWEL MUCOSA WITHOUT SIGNIFICANT PATHOLOGY. - PROMINENT BRUNNER'S GLANDS WITH EXTENSION INTO THE LAMINA PROPRIA.
Superficial Brunner's glands
DUODENUM, BIOPSY: - SMALL BOWEL MUCOSA WITH BRUNNER'S GLANDS THAT ARE FOCALLY SUPERFICIAL. - NO FINDINGS SUGGESTIVE OF CELIAC DISEASE. - NEGATIVE FOR ACTIVE INFLAMMATION. - NEGATIVE FOR DYSPLASIA.
Micro
The sections show small bowel mucosa and a small amount of submucosa. Brunner's glands are abundant and found focally in the lamina propria.
The epithelium matures appropriately. There is no increase in intraepithelial lymphocytes. No foveolar metaplasia of the epithelium is identified.
Helicobacter duodenitis
- Helicobacter is the most common cause of duodenitis.[8][9]
- Overall, Helicobacter is rare in the duodenum.
- Infection associated with gastric metaplasia.[10]
Weird stuff
Disaccharidases deficiency
General
- Common among asians.
- Includes: lactase, sucrase, and maltase.
- Lactase changes seen with mild histomorphologic changes.[11]
- Maltase and sucrase only affected in moderate and severe lesions.
Microscopic
Features:[11]
- Decreased villous-crypt ratio (mild to severe).
- +/-Inflammation (only in moderate and severe).
DDx:
- Celiac disease.[12]
Notes:
- May have normal histomorphology.[11]
Whipple disease
Microvillous inclusion disease
This rare disease presents very shortly after birth.
Tufting enteropathy
- AKA intestinal epithelial dysplasia.
General
- Genetic disease[13] - related to abnormal enterocytes (development and/or differentiation).
- Gene implicated: EPCAM.[14]
Microscopic
Features:[15]
- Villous atrophy
- Mononuclear cell infiltration of the lamina propria
- Abnormal surface enterocytes:
- Focal crowding -- resembling tufts.
Gangliocytic paraganglioma
- Abbreviated GP.
Pseudomelanosis duodeni
Tumours
Lymphoma
- Non-Hodgkin's lymphoma.
- Enteropathy-associated T-cell lymphoma (EATL) - due to celiac sprue.
- MALT lymphoma - common GI tract lymphoma.
- Mantle cell lymphoma.
- Diffuse large B cell lymphoma.
Note:
- Hodgkin's lymphoma does not arise in the GI tract.
Adenocarcinoma of the duodenum
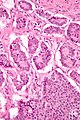
Duodenal neuroendocrine tumour
- Duodenal NET redirects here.
General
- Like neuroendocrine tumours elsewhere.
- Use of the term carcinoid is discouraged.[16][17][18]
Associations:
Microscopic
Features:[19]
- Usu. nests of cells - may be:
- Trabecular.
- Glandular - common in stomatostatin producing tumours.
- Stippled chromatin - (AKA salt-and-pepper chromatin, coarse chromatin).
- Classically subepithelial/mural.
- +/-Psammoma bodies - suggestive of somatostatinoma and NF1.[20]
DDx:
Images
Sign out
Duodenum, Biopsy: - Incidental neuroendocrine tumour, grade 1, see comment. - Background small bowel mucosa with Brunner's glands within normal limits. Comment: The tumour stains as follows: POSITIVE: AE1/AE3, CD56, synaptophysin. NEGATIVE: S-100, CD68. PROLIFERATION (Ki-67): <2%.
Ampullary tumours
General
- Individuals with high-grade dysplasia (on biopsy) are usually treated with a pancreaticoduodenectomy (Whipple procedure), as local resections have a very high recurrence rate.[21]
Microscopic
Features:
- See ampullary tumours.
DDx:
- Intraductal papillary mucinous tumour (IPMT) - a pancreatic tumour, see pancreas article.
- Invasive ductal carcinoma of the pancreas.
Sign out
- Ampullary carcinoma - has separate staging.
Traditional adenoma
- Duodenal adenoma redirects here.
General
- Strong association of familial adenomatous polyposis.
- In one series of 208 adenomas, almost 70% were from FAP patients.[22]
- Commonly found in association foveolar metaplasia - especially in sporadic cases ~60% of cases.
- In FAP ~30% of cases have foveolar metaplasia.[22]
- A colonscopy is recommended in individuals with nonampullary duodenal adenomas, as they are likely at increased risk of large bowel adenomas.[23]
Sign out
POLYP, DUODENUM, EXCISION: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
Alternate
Polyp (Nonampullary), Duodenum, Polypectomy:
- Tubular adenoma, NEGATIVE for high-grade dysplasia.
Comment:
A colonscopy is recommended if not done recently, as individual with nonampullary duodenal adenomas are likely at increased risk of large bowel adenomas.[1]
1. Therap Adv Gastroenterol. 2012 Mar; 5(2): 127138. doi: 10.1177/1756283X11429590
See also
References
- ↑ Agarwal S, Smereka P, Harpaz N, Cunningham-Rundles C, Mayer L (July 2010). "Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease". Inflamm Bowel Dis. doi:10.1002/ibd.21376. PMID 20629103.
- ↑ El-Zimaity. 18 October 2010.
- ↑ Serra S, Jani PA (November 2006). "An approach to duodenal biopsies". J. Clin. Pathol. 59 (11): 1133–50. doi:10.1136/jcp.2005.031260. PMC 1860495. PMID 16679353. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860495/?tool=pubmed.
- ↑ Tan, YM.; Wong, WK. (2002). "Giant Brunneroma as an unusual cause of upper gastrointestinal hemorrhage: report of a case.". Surg Today 32 (10): 910-2. doi:10.1007/s005950200179. PMID 12376792.
- ↑ 5.0 5.1 Lee, WC.; Yang, HW.; Lee, YJ.; Jung, SH.; Choi, GY.; Go, H.; Kim, A.; Cha, SW. (Jun 2008). "Brunner's gland hyperplasia: treatment of severe diffuse nodular hyperplasia mimicking a malignancy on pancreatic-duodenal area.". J Korean Med Sci 23 (3): 540-3. doi:10.3346/jkms.2008.23.3.540. PMID 18583897.
- ↑ 6.0 6.1 6.2 Patel, ND.; Levy, AD.; Mehrotra, AK.; Sobin, LH. (Sep 2006). "Brunner's gland hyperplasia and hamartoma: imaging features with clinicopathologic correlation.". AJR Am J Roentgenol 187 (3): 715-22. doi:10.2214/AJR.05.0564. PMID 16928936.
- ↑ Franzin, G.; Musola, R.; Ghidini, O.; Manfrini, C.; Fratton, A. (Dec 1985). "Nodular hyperplasia of Brunner's glands.". Gastrointest Endosc 31 (6): 374-8. PMID 4076734.
- ↑ URL: https://www.saintlukeskc.org/health-library/duodenitis. Accessed on: 2024 Feb 5.
- ↑ URL: https://www.webmd.com/digestive-disorders/what-is-duodenitis. Accessed on: 2024 Feb 5.
- ↑ Yang H, Dixon MF, Zuo J, Fong F, Zhou D, Corthésy I, Blum A (March 1995). "Helicobacter pylori infection and gastric metaplasia in the duodenum in China". J Clin Gastroenterol 20 (2): 110–2. doi:10.1097/00004836-199503000-00007. PMID 7769188.
- ↑ 11.0 11.1 11.2 Langman JM, Rowland R (July 1990). "Activity of duodenal disaccharidases in relation to normal and abnormal mucosal morphology". J. Clin. Pathol. 43 (7): 537–40. PMC 502575. PMID 2116456. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC502575/.
- ↑ Murray IA, Smith JA, Coupland K, Ansell ID, Long RG (February 2001). "Intestinal disaccharidase deficiency without villous atrophy may represent early celiac disease". Scand. J. Gastroenterol. 36 (2): 163–8. PMID 11252408.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 613217
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 185535
- ↑ Goulet O, Salomon J, Ruemmele F, de Serres NP, Brousse N (2007). "Intestinal epithelial dysplasia (tufting enteropathy)". Orphanet J Rare Dis 2: 20. doi:10.1186/1750-1172-2-20. PMC 1878471. PMID 17448233. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1878471/.
- ↑ Chetty, R. (Apr 2008). "Requiem for the term 'carcinoid tumour' in the gastrointestinal tract?". Can J Gastroenterol 22 (4): 357-8. PMID 18414708.
- ↑ Klöppel, G.; Perren, A.; Heitz, PU. (Apr 2004). "The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification.". Ann N Y Acad Sci 1014: 13-27. PMID 15153416.
- ↑ Klöppel G (July 2003). "[Neuroendocrine tumors of the gastrointestinal tract]" (in German). Pathologe 24 (4): 287–96. doi:10.1007/s00292-003-0636-7. PMID 14513276.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2011/SmallbowelNET_11protocol.pdf. Accessed on: 29 March 2012.
- ↑ Kim, JA.; Choi, WH.; Kim, CN.; Moon, YS.; Chang, SH.; Lee, HR. (Mar 2011). "Duodenal somatostatinoma: a case report and review.". Korean J Intern Med 26 (1): 103-7. doi:10.3904/kjim.2011.26.1.103. PMID 21437171.
- ↑ Meneghetti, AT.; Safadi, B.; Stewart, L.; Way, LW. (Dec 2005). "Local resection of ampullary tumors.". J Gastrointest Surg 9 (9): 1300-6. doi:10.1016/j.gassur.2005.08.031. PMID 16332486.
- ↑ 22.0 22.1 Rubio, CA. (Jun 2007). "Gastric duodenal metaplasia in duodenal adenomas.". J Clin Pathol 60 (6): 661-3. doi:10.1136/jcp.2006.039388. PMC 1955048. PMID 16837629. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1955048/.
- ↑ Lim, CH.; Cho, YS. (Jan 2016). "Nonampullary duodenal adenoma: Current understanding of its diagnosis, pathogenesis, and clinical management.". World J Gastroenterol 22 (2): 853-61. doi:10.3748/wjg.v22.i2.853. PMID 26811631.
External links
Review article(s)
- Serra S, Jani PA (November 2006). "An approach to duodenal biopsies". J. Clin. Pathol. 59 (11): 1133–50. doi:10.1136/jcp.2005.031260. PMC 1860495. PMID 16679353. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860495/?tool=pubmed.