Meningioma
Jump to navigation
Jump to search
Meningioma a very common tumour in neuropathology.
| Meningioma | |
|---|---|
| Diagnosis in short | |
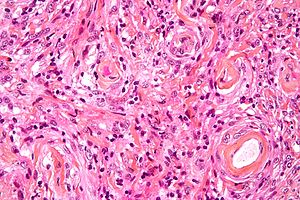 Meningioma. HPS stain. | |
|
| |
| LM | whorled appearance, calcification - psammomatous, +/-nuclear pseudoinclusions |
| Subtypes | Grade I (meningothelial, fibrous, transistional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, metaplastic), Grade II (invasive, clear cell, chordoid), Grade III (papillary, rhabdoid) |
| LM DDx | schwannoma, solitary fibrous tumour, hemangiopericytoma, others |
| IHC | EMA +ve, keratins usu. -ve, CD34 -ve/+ve, S-100 -ve (usu.), PR +ve (-ve in more aggressive ones) |
| Site | see CNS tumours |
|
| |
| Syndromes | neurofibromatosis 2, nevoid basal cell carcinoma syndrome |
|
| |
| Clinical history | +/-radiation |
| Prevalence | common |
| Radiology | extra-axial, intradural lesion, dural tail sign (on MRI) |
| Prognosis | usually benign, dependent on grade |
| Clin. DDx | dependent on site - see CNS tumours |
| Treatment | surgical removal |
General
Prevalence
Prognosis
- Most are benign - usu. a good prognosis.
- May be malignant - bad prognosis.
Genetics
- May be seen in genetic disorders such as:
- Neurofibromatosis 2 (NF2).[3]
- Nevoid basal cell carcinoma syndrome (Gorlin syndrome).[4][5]
Quick overview
| Name | Histologic criteria | Subtypes | Image |
|---|---|---|---|
| Classic, WHO I | less then 4 mit/10 HPF and no atypia | meningeothelial, fibroblastic, transitional, psammomatous, angiomatous, microcytsic, secretory, lymphoplasmacyte-rich, metaplastic | |
| Atypical, WHO II | brain invasion, 4 or more mit/10 HPF, or 3 of the following: necrosis, increased cellularity, high nuc:cyto ratio, nucleoli, sheeting | chordoid, clear cell | |
| Anaplastic, WHO III | 20 or more mitoses/10 HPF, morphologiy similiar to carcinoma or sarcoma | rhabdoid, papillary |
Gross/Radiology
- Extra-axial, intradural.
- Can be extradural - very rare.[6]
- Dural tail sign (DTS) on MRI.[7][8]
- +/-Hyperostosis.
- Associated with invasion into the skull in ~20% of cases.[11]
Microscopic
Features (memory device WCN):
- Whorled appearance - key feature.
- Calcification, psammomatous (target-like appearance; (tight) onion skin).
- +/-Nuclear pseudoinclusions - focal nuclear clearing with a sharp interface to unremarkable chromatin.
Notes:
- May involute into benign sclerotic tissue.[12]
- Thick-walled blood vessels -> think schwannoma.
DDx:
- Schwannoma - especially at CP angle.
- Solitary fibrous tumour.
- Hemangiopericytoma.
- Others - see subtypes.
Images
www:
Morphologic subtypes
- Many subtypes exist.[13]
- The histologic subtypes generally don't have much prognostic significance.
- Some subtypes are high grade by definition; also see histologic grading.
Grade I
Meningothelial meningioma
- Most common.
Microscopic:
- Syncytial, nuclear clearing (pseudoinclusions).
- Whorls, Onion bulb formations.
- Few psammoma bodies.
Molecular:
- AKT E17K mutations.[14]
Fibrous meningioma
- AKA fibroblastic meningioma.
- Not collagen... but looks like it.
- It is really laminin or fibronectin.
- Spindle cells in parallel bundles.
- Few to none whorl formations.
Transitional meningioma
- AKA mixed.
- Common.
- Lobular and fasicular growth patterns coexist.
- Usu. a mixture of meningeothelial and fibromatous meningioma
Psammomatous meningioma
Microscopic:
- Psammoma bodies dominate over tumor cells.
- Irregular calcifications (confluent psammoma bodies).
- Usually found in spinal cord.
Angiomatous meningioma
- AKA vascular.
- May bleed like stink.
- May show extensive edema.
- Hyalinized vessels dominate over tumor cells.
- Degenerative nuclear atypia.
DDx:
- Vascular malformatons
- Hemangioblastoma
Microcystic meningioma
Microscopic:
- Cystic appearance.
- Increased cytologic pleomorphism of the elongated cells.
DDx:
- Clear cell meningioma
- Hemangioblastoma
Secretory meningioma
- Associated with brain edema; may have a worse outcome.
Microscopic:[15]
Molecular:
- Combined KLF4 K409Q and TRAF7 mutations.[16]
DDx:
- Metastatic mucinous adenocarcinoma.
- Pituitary adenoma
Images:
Lymphoplasmacyte-rich meningioma
Microscopic:
- Lymphocytes.
- Plasma cells.
Images:
- Lymphoplasmacyte-rich meningioma - case 1 - several images (upmc.edu).
- Lymphoplasmacyte-rich meningioma - case 2 - several images (upmc.edu).
- Lymphoplasmacyte-rich meningioma - case 3 - several images (upmc.edu).
Metaplastic meningioma
- Much talked about... but very rare.
Microscopic:
- Cartilage or bone formation.
Grade II
Brain invasive meningioma
- Invades the brain.
Images:
Clear cell meningioma
Epidemiology:
- Usu. spinal cord.[18]
Microscopic:
- Clear cells - contain glycogen (PAS +ve).
Molecular:
- SMARCE1 mutations.[19]
Images:
Chordoid meningioma
- Chordoma-like.
Microscopic:
- Myxoid appearance.
Image:
Grade III
Papillary meningioma
Microscopic:
- discohesive meningothelial tumour cells around a fibrovascular core.
- perivascular pseudorosettes.
Rhabdoid meningioma
Microscopic:
- Rhabdoid appearance (abundant cytoplasm).
- Cross-striations.
www:
- Rhabdoid meningioma - case 1 - several images (upmc.edu).
- Rhabdoid meningioma - case 2 - several images (upmc.edu).
Histologic grading
Grading:[13]
- Grade 1:
- Low mitotic rate (< 4 mitoses/10 HPF - for whatever HPF means, see HPFitis).
- Excludes clear cell, chordoid, papillary, and rhabdoid subtypes.
- Grade 2 (either #1, #2 or #3):
- Brain-invasive meningioma.
- Invasion of meningioma into brain.
- Meninogioma with entraped GFAP +ve tissue.
- Invasion of meningioma into brain.
- Atypical meningioma (by histomorphology) - either A or B.
- A. Intermediate mitotic rate (>= 4 mitoses/10 HPF - for whatever HPF means, see HPFitis.)
- B. Three of the following five features:
- Clear cell or chordoid subtype.
- Brain-invasive meningioma.
- Grade 3 (either of the following):
- High mitotic rate (>=20 mitoses/10 HPF - for whatever HPF means, see HPFitis.)
- "Frank anaplasia"; marked nuclear atypia.
- Papillary or rhabdoid subtype.
Notes:
- Grade II soft criteria memory device HMNs: hypercellular, macronucleoli, NC ratio increased, necrosis, sheeting.
IHC
- EMA +ve.[20]
- Other CKs usually -ve.
Molecular
Non-syndromal meningiomas may show SMO and AKT mutations.[21]
Several inherited diseases are associated with meningiomas:
- Neurofibromatosis type II[22]
- Germline SMARCE1 and SMARCB1 mutations[23][24]
- Loss of SUFU (SHH-Pathway)[25]
DDx of meningioma & IHC[26]
- S-100 +ve - schwannoma.
- +ve in ~80% of fibrous meningiomas.
- CD34 +ve - solitary fibrous tumour.
- +ve in ~60% of fibrous meningiomas.
- STAT6 nuclear +ve: solitary fibrous tumour.
- EMA +ve in ~30% of hemangiopericytoma.
- Claudin-1 - new kid on the block: +ve in meningioma, but low sensitivity.
- Progesterone receptor: +ve in mostly grade I and meningeothelial tumors.[27]
- Pulmonary meningothelial-like nodule
A standard work-up
See also
References
- ↑ Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, TJ.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, DC. et al. (Oct 2014). "Meningiomas: knowledge base, treatment outcomes, and uncertainties. A RANO review.". J Neurosurg: 1-20. doi:10.3171/2014.7.JNS131644. PMID 25343186.
- ↑ Baldi, I.; Engelhardt, J.; Bonnet, C.; Bauchet, L.; Berteaud, E.; Grüber, A.; Loiseau, H. (Sep 2014). "Epidemiology of meningiomas.". Neurochirurgie. doi:10.1016/j.neuchi.2014.05.006. PMID 25249493.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/neurotest/Q13-Ans.htm. Accessed on: 26 October 2010.
- ↑ Kimonis, VE.; Mehta, SG.; Digiovanna, JJ.; Bale, SJ.; Pastakia, B.. "Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome.". Genet Med 6 (6): 495-502. doi:10.109701.GIM.0000145045.17711.1C. PMID 15545745.
- ↑ Lee, CW.; Tan, TC. (Feb 2014). "Meningioma associated with Gorlin's syndrome.". J Clin Neurosci 21 (2): 349-50. doi:10.1016/j.jocn.2013.02.033. PMID 24100109.
- ↑ URL: http://path.upmc.edu/cases/case702.html. Accessed on: 2 February 2012.
- ↑ Ikeda, D.; Chiocca, EA. (Oct 2012). "Editorial: dural tail sign.". J Neurosurg 117 (4): 643-4. doi:10.3171/2012.2.JNS12266. PMID 22839655.
- ↑ Wen, M.; Jung, S.; Moon, KS.; Pei, J.; Lee, KH.; Jin, SG.; Li, SY.; Ryu, HH. (Dec 2014). "Immunohistochemical profile of the dural tail in intracranial meningiomas.". Acta Neurochir (Wien) 156 (12): 2263-73. doi:10.1007/s00701-014-2216-4. PMID 25238986.
- ↑ Aoki, S.; Sasaki, Y.; Machida, T.; Tanioka, H.. "Contrast-enhanced MR images in patients with meningioma: importance of enhancement of the dura adjacent to the tumor.". AJNR Am J Neuroradiol 11 (5): 935-8. PMID 2120998.
- ↑ Qi, ST.; Liu, Y.; Pan, J.; Chotai, S.; Fang, LX. (Oct 2012). "A radiopathological classification of dural tail sign of meningiomas.". J Neurosurg 117 (4): 645-53. doi:10.3171/2012.6.JNS111987. PMID 22839654.
- ↑ Goyal, N.; Kakkar, A.; Sarkar, C.; Agrawal, D.. "Does bony hyperostosis in intracranial meningioma signify tumor invasion? A radio-pathologic study.". Neurol India 60 (1): 50-4. doi:10.4103/0028-3886.93589. PMID 22406780.
- ↑ URL: http://radiographics.rsna.org/content/23/3/785.long. Accessed on: 3 November 2010.
- ↑ 13.0 13.1 Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 194. ISBN 978-0443069826.
- ↑ Sahm, F.; Bissel, J.; Koelsche, C.; Schweizer, L.; Capper, D.; Reuss, D.; Böhmer, K.; Lass, U. et al. (Nov 2013). "AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry.". Acta Neuropathol 126 (5): 757-62. doi:10.1007/s00401-013-1187-5. PMID 24096618.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/Com04/Com405-1-Diss.htm. Accessed on: 12 October 2011.
- ↑ Reuss, DE.; Piro, RM.; Jones, DT.; Simon, M.; Ketter, R.; Kool, M.; Becker, A.; Sahm, F. et al. (Mar 2013). "Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations.". Acta Neuropathol 125 (3): 351-8. doi:10.1007/s00401-013-1093-x. PMID 23404370.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/Com04/Com405-1-Diss.htm. Accessed on: 3 January 2012.
- ↑ Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 200. ISBN 978-0443069826.
- ↑ Smith, MJ.; Wallace, AJ.; Bennett, C.; Hasselblatt, M.; Elert-Dobkowska, E.; Evans, LT.; Hickey, WF.; van Hoff, J. et al. (Dec 2014). "Germline SMARCE1 mutations predispose to both spinal and cranial clear cell meningiomas.". J Pathol 234 (4): 436-40. doi:10.1002/path.4427. PMID 25143307.
- ↑ Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 13. ISBN 978-0443069826.
- ↑ Clark, VE.; Erson-Omay, EZ.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avşar, T.; Li, J. et al. (Mar 2013). "Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO.". Science 339 (6123): 1077-80. doi:10.1126/science.1233009. PMID 23348505.
- ↑ Fontaine, B.; Rouleau, GA.; Seizinger, BR.; Menon, AG.; Jewell, AF.; Martuza, RL.; Gusella, JF. (1991). "Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuroma and meningioma).". Ann N Y Acad Sci 615: 338-43. PMID 2039155.
- ↑ Smith, MJ.; O'Sullivan, J.; Bhaskar, SS.; Hadfield, KD.; Poke, G.; Caird, J.; Sharif, S.; Eccles, D. et al. (Mar 2013). "Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas.". Nat Genet 45 (3): 295-8. doi:10.1038/ng.2552. PMID 23377182.
- ↑ van den Munckhof, P.; Christiaans, I.; Kenter, SB.; Baas, F.; Hulsebos, TJ. (Feb 2012). "Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri.". Neurogenetics 13 (1): 1-7. doi:10.1007/s10048-011-0300-y. PMID 22038540.
- ↑ Aavikko, M.; Li, SP.; Saarinen, S.; Alhopuro, P.; Kaasinen, E.; Morgunova, E.; Li, Y.; Vesanen, K. et al. (Sep 2012). "Loss of SUFU function in familial multiple meningioma.". Am J Hum Genet 91 (3): 520-6. doi:10.1016/j.ajhg.2012.07.015. PMID 22958902.
- ↑ Hahn HP, Bundock EA, Hornick JL (February 2006). "Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics". Am. J. Clin. Pathol. 125 (2): 203–8. doi:10.1309/G659-FVVB-MG7U-4RPQ. PMID 16393681. http://ajcp.ascpjournals.org/content/125/2/203.full.pdf.
- ↑ Grunberg, SM. (1991). "The role of progesterone receptors in meningioma.". Cancer Treat Res 58: 127-37. PMID 1683782.
- ↑ Croul, SE. 8 November 2010.
- ↑ Takei, H.; Buckleair, LW.; Powell, SZ. (Feb 2008). "Immunohistochemical expression of apoptosis regulating proteins and sex hormone receptors in meningiomas.". Neuropathology 28 (1): 62-8. doi:10.1111/j.1440-1789.2007.00852.x. PMID 18021195.
- ↑ Tao, Y.; Liang, G.; Li, Z.; Wang, Y.; Wu, A.; Wang, H.; Lu, Y.; Liu, Z. et al. (May 2012). "Clinical features and immunohistochemical expression levels of androgen, estrogen, progesterone and Ki-67 receptors in relationship with gross-total resected meningiomas relapse.". Br J Neurosurg. doi:10.3109/02688697.2012.685780. PMID 22616825.