Difference between revisions of "Liver neoplasms"
(→Image) |
|||
| Line 581: | Line 581: | ||
*Metastases are typically negative for ''HepPar-1''. | *Metastases are typically negative for ''HepPar-1''. | ||
**HepPar-1 (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.<ref name=pmid12502967>The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.</ref> | **HepPar-1 (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.<ref name=pmid12502967>The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.</ref> | ||
===Sign out=== | |||
<pre> | |||
LIVER, PORTION OF SEGMENTS 2 AND 3, RESECTION: | |||
- METATSTATIC ADENOACARCINOMA. | |||
-- RESECTION MARGIN CLEARANCE 2 MM. | |||
- LIVER STEATOSIS, MILD. | |||
</pre> | |||
====Micro==== | |||
The section show liver parenchyma with an invasive adenocarcinoma. The adenocarcinoma | |||
has well formed glands with dirty necrosis. The nuclei are appear crowded and | |||
have an ellipsoid shape. Focally, zones of necrosis are present. See background liver. | |||
BACKGROUND LIVER (BASED ON H&E ONLY) | |||
Fibrosis: not identified.<br> | |||
Fibrous septa: absent.<br> | |||
Septa with curved contours: absent.<br> | |||
Large droplet steatosis (% of hepatocytes): mild (20%).<br> | |||
Ballooning of hepatocytes: not identified.<br> | |||
Mallory-Denk bodies: not identified.<br> | |||
Portal inflammation: present, mild.<br> | |||
Interface activity: not identified.<br> | |||
Lobular necroinflammation: not identified.<br> | |||
Ducts: present in normal numbers.<br> | |||
Duct injury: not identified.<br> | |||
Ductular reaction: not identified.<br> | |||
Cholestasis: present peritumoural, otherwise absent.<br> | |||
Terminal hepatic venules: present.<br> | |||
Ground glass cells with routine stains: not identified. | |||
=See also= | =See also= | ||
Revision as of 08:07, 5 August 2013
This article examines liver neoplasms and pre-malignant lesions of the liver. In North America, most malignant liver lesions are metastases.
This article focuses on primary malignancies of the liver, neoplastic liver lesions, and biliary malignancies. It only briefly discusses metastatic lesions. An introduction to liver pathology is in the liver article. Medical liver disease is dealt with in the medical liver disease article.
Overview
Dysplasic lesions of the liver
Types:[1]
- "Large cell dysplasia" (AKA large cell change) - not considered a precursor for HCC, not considered a dysplasia.[2]
- Small liver cell dysplasia (AKA small cell dysplasia).
- Low grade dysplasia.
- High grade dysplasia.
Neoplastic lesions
Malignant lesions of the liver
- Hepatocellular carcinoma (HCC) - most common malignant liver primary in adults.
- Hepatoblastoma - malignant liver primary in children.
- Intrahepatic cholangiocarcinoma (ICC).[3]
- Combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma (CHC).
Lesions that arise in a non-cirrhotic liver
Hepatocellular:
Other:
Tabular comparison
Precursors
Features of HCC & its precursors - generated from DCHH[4] and STC:
| Features | SLCD | Low-grade dysplasia | High-grade dysplasia | HCC |
| Plate thickness | <3 cells | <=2 cells | <=3 cells, usu. >2 cells | >3 cells |
| Reticulin (stain) | intact chicken wire | intact chicken wire | intact chicken wire | damaged chicken wire |
| Nuclear changes | nuc. enlargement, hyperchromasia |
+/- atypia (???) | marked atypia | +/- incr. NCR, +/-irreg. nuc. contour |
| Cytoplasmic change | hyperchromasia, decr. as cell size preserved |
none (???) | +/- basophilia | variable (lighter vs. hyperchromasia) |
| Portal tracts | ? | loss of portal tracts | loss of portal tracts | loss of portal tracts |
| Management | follow ??? | follow | ablate | ablate/surgery |
Abbreviations:
- SLCD = small liver cell dysplasia.
Notes:
- Large cell dysplasia:
- Cell size ~ 2x normal, NC ratio ~ normal.
- SLCD:
- Cell size ~ 1/2x normal, NC ratio - increased.
Hepatic tumours
Benign:
| Entity | Gross | Microscopic | IHC/stains | Other | Images |
|---|---|---|---|---|---|
| Hepatic hemangioma | similar to normal liver parenchyma, red (hemorrhagic), well-circumscribed | spaces lined by benign endothelial cells | CD31+ (???) | - | gross (rsna.org) |
| Focal nodular hyperplasia | central scar, large vessels, usu. well-circumscribed | large arteries, unpaired arteries, bile duct proliferation | usu. diagnosed by imaging | gross (rsna.org) | |
| Hepatocellular adenoma | subcapsular, well-circumscribed | loss of portal tracts, nuclear glycogenation | reticulin - liver plate thickness <= 3 | background not cirrhotic, assoc. OCP | gross (mda-sy.com)[5] |
Malignant:
| Entity | Gross | Microscopic | IHC/stains | Other | Images |
|---|---|---|---|---|---|
| Liver metastasis | multiple, white lesions | variable, usu. tubular (glandular) with pseudostratified hyperchromatic nuclei | CK7-, CK20-, HepPar-1-, CK19- | colorectal carcinoma most common | |
| Hepatocellular carcinoma | poorly circumscribed, +/-necrosis, +/-hemorrhage | loss of portal tracts, unpaired arteries, +/-nuclear atypia | reticulin - liver plate thickness > 3 | background often cirrhotic | |
| Cholangiocarcinoma | cauliflower-like outline, white, classically solitary, no cirrhosis | tubular architecture and mild nuclear atypia (adenocarcinoma), desmoplastic stroma | CK7+, CK19+ | background usu. not cirrhotic |
Dysplasia of the liver
Small liver cell dysplasia
- Abbreviated SLCD.
- AKA small cell dysplasia.
General
- Considered a precursor to HCC.
- Frequently found in livers with HCC - when compared to livers without HCC.[6]
Microscopic
Features:[7]
- Cells similar in size to normal hepatocytes.
- Name derived from the fact that there is also an entity that was called large cell dysplasia (AKA large cell change).
- Increased NC ratio - "more blue".
- Mild nuclear and cytoplasmic hyperchromatism.
Notes:
- Normal hepatic architecture (main differentiator from HCC).
- Remember "... blue is bad".
Micrograph:
Low grade dysplasia
Microscopic
- Uniform cells - "noticeably different from normal".[8]
- Changes in nuclear size, irregular nuclear contour and/or changes in cytoplasm staining.
- Loss of portal tracts.
- Irregular margin.
Notes:
- DCHH describes LGD as: "normal hepatocytes in plates [of normal thickness]".[4]
DDx:
- Nodular regenerative hyperplasia - lacks: compressed rim of cells, central portal tract.[4]
High grade dysplasia
General
- "Bader" version of low grade dyplasia.
Microscopic
Features - in addition to those of low grade dysplasia:[4]
- Liver plate >2 cells thick.
- Significant nuclear atypia.
- Basophilic cytoplasm.
Image:
Benign hepatic neoplasms
Bile duct adenoma
- Should not be confused with bile duct hamartoma.
General
- Benign.
- Important as it can be misdiagnosed as cancer.
Microscopic
Features:
- Disordered bile ducts within in a fibrotic stroma.
- No (yellow) bile within, as these lesions do not have a connection to the biliary tree.
- +/-Lymphocytic cuff.
Negatives:
- No mitotic activity.
- No necrosis.
DDx:
- Cholangiocarcinoma.
- Metastatic adenocarcinoma.
- Bile duct hamartoma (von Meyenburg complex).
Image:
Hepatic adenoma
- AKA hepatocellular adenoma, abbreviated HCA.
General
- Grow under the influence of sex hormones.
- Associated with oral contraceptive pill (OCP) use[9][10] - may regress with discontinuation.
- May rupture in pregnancy.
- Usually diagnosed by radiology.
Gross
Features:[11]
- Often subcapsular location.
- Well circumscribed, but not encapsulated.
Microscopic
Features:
- Sheets or cords of cells with mild variation of cell and nuclear size.[12]
- Cords of cells upto 3 cells thick.[13]
- Cells may have cytoplasmic clearing due to glycogen or be pale - obvious if seen.
- Vascular - large arteries, dilated thin-walled veins.
Negatives:
- No bile ducts.
- No portal tracts.
- No cirrhosis! If cirrhosis is present it isn't a hepatic adenoma - important.
DDx:
- Well-differentiated hepatocellular carcinoma.[14]
- Hepatic adenoma is differentiated from well-differentiated HCC by its architecture; adenomas have cords of cells upto 3 cells thick & have preserved reticulin architecture.
- Focal nodular hyperplasia.
Images
www:
- Hepatic adenoma (ajronline.org).
- Series of liver micrographs including hepatic adenoma (radiographics.rsna.org).
Subclassification
Based on molecular changes:[15][16]
- Inflammatory hepatic adenoma.
- AKA telangiectatic adenoma.[17]
- Associated with obesity.[citation needed]
- Hepatocyte nuclear factor 1 alpha-mutated hepatic adenoma.
- Inactivating mutation.
- Beta-catenin-mutated hepatic adenoma
- Activating mutation.
- Unclassified hepatic adenoma.
Note:
- Beta-catenin is considered an oncogene.
IHC
- AFP -ve. (???)
- HNF1alpha +ve/-ve.
- Beta-catenin +ve/-ve.
Hepatobiliary mucinous cystadenoma
- AKA biliary cystadenoma.
General
- Benign neoplasm.
- May transform into a malignancy.[18]
Microscopic
Features:
- Cystic spaces lined by a mucinous epithelium (simple columnar epithelium with a clear cytoplasm).
Note:
- Similar to pancreatic mucinous cystadenoma.
Malignant hepatic neoplasms
In North America, the most common malignant liver tumour is metastases.
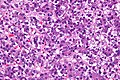
Hepatoblastoma
General
- Most common liver cancer in children.[12][19]
- Rare in adolescents and adults.
- Age of diagnosis usu. ~1 year old; most less than 3 years old.
- Surgical biopsy; core needle biopsy not done as as lesion is vascular.
Associations:
Clinical:
- Usually present with hepatomegaly.
- High AFP.[21]
Microscopic
Features:
- Small round cell tumour.
- Fetal hepatocytes ~ 1:3 NC ratio, eosinophilic cytoplasm.
- +/-Mesenchymal component
- Immature fibrous tissue, osteoid or cartilage.
DDx:
- Small round cell tumours.
- Teratoma.
- Hepatocellular carcinoma - separated based on histomorphology alone.
Images
Subtypes
- Six histologic subtypes - that are subdivided into two groups:[22]
- Epithelial type:
- Fetal pattern.
- Embryonal and fetal pattern.
- Macrotrabecular pattern.
- May mimic hepatocellular carcinoma histologically.[23]
- Small cell undifferentiated pattern.
- Poor prognosis.
- Mixed epithelial and mesenchymal type:
- With teratoid features.
- Without teratoid features.
- Epithelial type:
IHC
- Alpha-fetoprotein +ve.
- Hepatocyte specific antigen +ve esp. in fetal component.[24]
- Beta-catenin +ve (cytoplasmic and nuclear).[24]
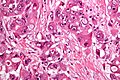
Hepatocellular carcinoma
- Commonly abbreviated HCC.
General
Clinical:
- Serum AFP elevated - in approx. 50% of patients.[25]
- Treatments: RFA (radiofrequency ablation), ethanol ablation, liver resection, liver transplant.[26]
- Mean survival at time of diagnosis ~6 months.[26]
Epidemiology:
- Highest where prevalence of hepatitis B virus (HBV) is high.[27]
- HCC generally arises in the setting of cirrhosis.
- Cirrhosis may be regressed and therefore not appreciated.
HCCs without cirrhosis:
- Hepatitis B virus.[27]
- Hemochromatosis.
- Fibrolamellar HCC.
- Chronic alcoholism.
- Hepatitis C virus (HCV) - chronic infection.
- Hepatitis B virus (HBV) - chronic infection.
- Aflatoxins (food contaminant - mould).[26]
- Hereditary tyrosinemia.
- Hereditary hemochromatosis.
Gross
Features:[29]
- Unifocal, multifocal or diffusely infiltrative.
- Pale in relation to surrounding liver or green (due to bile secretion).
Microscopic
Requirements:[32]
- Architectural changes.
- Liver plate more than 3 cells thick - key feature.
- Loss of reticulin scaffold - incomplete loss is considered significant.
- CD34+ staining cells, suggesting loss of epithelial cells that form the sinusoids.
- Loss of structures seen in a normal liver lobule (bile ductules, portal triad).
- Invasion of the portal tract - useful in well-diff. lesions.[33]
Additional findings:[34]
- Nuclear changes.
- Increased NC ratio - key feature if present.
- Nuclear hyperchromasia.
- Abnormal nuclear contour.
- Mitoses.
- Cytoplasmic changes.
- Cytoplasmic hyperchromasia, clearing or lighter staining.
Varied architecture - may be:[35]
- Pseudoglandular - can be confused with adenocarcinoma.
- Trabecular.
- Fibrolamellar.
- Solid.
Notes:
- HCC with trabecular morphology has some resemblance to normal liver - but has extra cells.
- Fibrolamellar - better prognosis, classically in young adults.
- Stroma is usually scant.[36]
ASIDE:
- Trabecula = little beam.
DDx:
- Cholangiocarcinoma.
- Combined HCC-CC.[37]
Images
Fibrolamellar hepatocellular carcinoma
- Abbreviated fibrolamellar HCC, FL-HCC, and FHCC.
General
- Rare variant.
- Classically afflicts younger patients.
- Mean age at onset ~27 years in one study.[38]
- Individuals usually do not have the classic risk factors for HCC, i.e. no cirrhosis, no hepatitis.[38]
Clinical:
- AFP usu. not elevated.[38]
Microscopic
Features:[39]
- Large polygonal tumours cells with:
- Graunular eosinophilic cytoplasm.
- Low NC ratio.[40]
- Layered dense collagen bundles.
DDx:
Note:
- If conventional HCC is seen focally within the tumour, it is conventional HCC.
Images
Sclerosing HCC
Features:
- Fibrosis. (???)
Notes:
- Seen in non-cirrhotic livers.
Grading
Edmondson-Steiner grading system:[41][42]
- Well-differentiated.
- Some say "it cannot be diagnosed on biopsy,"[43] as it cannot be reliably differentiated from a regenerative nodule.
- Moderately differentiated.
- Round, regular nuclei, some hyperchromatism, nucleoli present, increase NC ratio.
- Poor differentiated.
- Very prominent nucleoli, pronounced nuclear irregularity.
- Undifferentiated.
- Anaplastic giant cells.
Simplified description - based on MacSween:[42]
- Well-differentiated = cytologically near normal.
- Moderate = looks like a cancer, small nucleoli.
- Poor = bad cancer, raisin-like (irregular) nuclear membrane, large nucleoli (~1/3 of nucleus).
- Undifferentiated = death on a slide, huge cells (3-4x the size of other cells).
IHC
- CD34 +ve sinusoids; sinusoids in normal liver are CD34 -ve.
- HepPar-1 +ve; may be neg. in high grade tumours.
- AFP +ve; may be neg. even if the serum AFP is elevated.
- CK8/18 +ve.[44]
- Glypican-3 +ve (cytoplasmic, granular cytoplasmic or membranous).[3]
Bile canaliculi:
Image:
Sign out
Negative core biopsy
LIVER CORE, BIOPSY: - CIRRHOSIS. - HEPATOCYTE CYTOLOGY WITHIN NORMAL LIMITS.
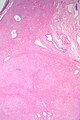
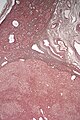
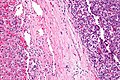
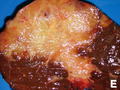
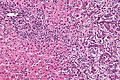
Cholangiocarcinoma
General
- Malignancy of the biliary tree.
- May be intrahepatic, i.e. intrahepatic cholangiocarcinoma (abbreviated ICC), or extrahepatic.
Epidemiology
- Rare - approximately 1/5 the incidence of HCC.[49]
- More common among asians.
Risks:
- Infection - liver flukes (endemic to Southeast Asia):
- Caroli disease - rare congenital disease.[53]
- Primary sclerosing cholangitis - may be assoc. with inflammatory bowel disease (IBD), esp. ulcerative colitis (UC).
Gross
- Classically one large mass - outline described as cauliflower-like.[54]
- May have satellite nodules.
Image
Microscopic
Features:[55]
- Usually an adenocarcinoma, i.e. gland forming with:
- Cuboidal or columnar mucin producing cells, and
- A dense fibrous (desmoplastic) stroma.
Notes:
- Biliary stents lead to reactive changes,[56] these can be confused for malignancy. One must always check whether a biliary stent was in situ at time of biopsy.[57]
- Usually abundant desmoplasia, ergo hard to get good, i.e. diagnositic, endoluminal brushing specimens.[58]
- May have hyaline inclusions.[59]
DDx:
- Metastatic adenocarcinoma.
- Fulminant hepatic necrosis.
- Bile ducts usu. left behind... look like well-differentiated adenocarcinoma.
- Bile duct adenoma.
- No necrosis, no mitotic activity, no significant nuclear pleomorphism.
Images
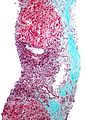
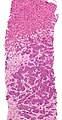
Cholangiocarcinoma - low mag. Shows the typical desmoplastic stroma. (WC/Nephron)
www:
- Cholangiocarcinoma & liver flukes - several images (upmc.edu).
- Cholangiocarcinoma - several images (upmc.edu).
IHC
Classic IHC pattern:[60]
- CK7 +ve.
- CK20 +ve/-ve.
- HepPar-1 -ve.
- AFP -ve.[61]
ICC vs. HCC:[62]
- ICC: CK19 (92.5%), MUC-1 (73.8%) +ve.
- HCC: HepPar-1 (85.6%), CD34 (87.8%) +ve.
HCC vs. ICC:[63]
- TTF-1: ~90-100% +ve (cytoplasmic) in HCC vs. ~10% in cholangiocarcinoma.
Hepatic angiosarcoma
- AKA angiosarcoma of the liver.
General
- Liver angiosarcomas are associated with vinyl chloride exposure.[64]
Microscopic
Features:
- Atypical endothelial cells - may be subtle.
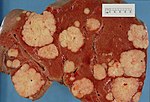
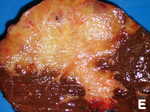
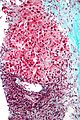
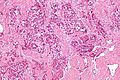
Hepatic metastasis
- AKA liver metastases.
General
- Metastases are very common - often from the gastrointestinal tract, e.g. colorectal cancer.
- Most liver masses in are not biopsied... as a primary lesion is evident.[65]
- Dependent on the extent of disease, CRC metastatic to the liver may be curable with a liver resection.
- It is important to consider germ cell tumours in the DDx as these may be curable with chemotherapy.
- Clear cell variant of HCC may be misdiagnosed as metastatic clear cell carcinoma.
- Interhepatic cholangiocarcinoma is an adenocarcinoma - it may look like a metastatic lesion.
Further reading:
- Anders, RA.; Kamel, IR. (May 2007). "Biopsy considerations in the diagnosis of hepatic masses.". Clin Gastroenterol Hepatol 5 (5): 541-4. doi:10.1016/j.cgh.2007.02.028. PMID 17478344.
Gross pathology/radiology
- Multifocal or solitary.
Microscopic
Features:
- Histologic features are dependent on primary and degree of differentiation.
The classic liver metastasis (colorectal carcinoma):
- Gland forming columnar shaped cells with pseudostratified hyperchromatic cigar-shaped nuclei.
Image
IHC
- Metastases are typically negative for HepPar-1.
- HepPar-1 (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.[66]
Sign out
LIVER, PORTION OF SEGMENTS 2 AND 3, RESECTION: - METATSTATIC ADENOACARCINOMA. -- RESECTION MARGIN CLEARANCE 2 MM. - LIVER STEATOSIS, MILD.
Micro
The section show liver parenchyma with an invasive adenocarcinoma. The adenocarcinoma has well formed glands with dirty necrosis. The nuclei are appear crowded and have an ellipsoid shape. Focally, zones of necrosis are present. See background liver.
BACKGROUND LIVER (BASED ON H&E ONLY)
Fibrosis: not identified.
Fibrous septa: absent.
Septa with curved contours: absent.
Large droplet steatosis (% of hepatocytes): mild (20%).
Ballooning of hepatocytes: not identified.
Mallory-Denk bodies: not identified.
Portal inflammation: present, mild.
Interface activity: not identified.
Lobular necroinflammation: not identified.
Ducts: present in normal numbers.
Duct injury: not identified.
Ductular reaction: not identified.
Cholestasis: present peritumoural, otherwise absent.
Terminal hepatic venules: present.
Ground glass cells with routine stains: not identified.
See also
References
- ↑ STC. S.30-37, 19 Jan 2009.
- ↑ Park, YN.; Roncalli, M. (Nov 2006). "Large liver cell dysplasia: a controversial entity.". J Hepatol 45 (5): 734-43. doi:10.1016/j.jhep.2006.08.002. PMID 16982109.
- ↑ 3.0 3.1 Shirakawa, H.; Kuronuma, T.; Nishimura, Y.; Hasebe, T.; Nakano, M.; Gotohda, N.; Takahashi, S.; Nakagohri, T. et al. (Mar 2009). "Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer.". Int J Oncol 34 (3): 649-56. PMID 19212669. http://www.spandidos-publications.com/serveFile/ijo_34_3_649_PDF.pdf?type=article&article_id=ijo_34_3_649&item=PDF.
- ↑ 4.0 4.1 4.2 4.3 Tadrous, Paul.J. Diagnostic Criteria Handbook in Histopathology: A Surgical Pathology Vade Mecum (1st ed.). Wiley. pp. 170-1. ISBN 978-0470519035.
- ↑ URL: http://www.mda-sy.com/vb/showthread.php?p=5083&langid=1. Accessed on: 16 February 2012.
- ↑ Szczepański, W. (1997). "Liver cell dysplasia in liver cirrhosis and hepatocellular carcinoma.". Pol J Pathol 48 (3): 147-57. PMID 9401407.
- ↑ STC S.32, 19 Jan 2009.
- ↑ STC - 19 Jan 2009. (???)
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 221. ISBN 978-0781765275.
- ↑ Rooks, JB.; Ory, HW.; Ishak, KG.; Strauss, LT.; Greenspan, JR.; Hill, AP.; Tyler, CW. (Aug 1979). "Epidemiology of hepatocellular adenoma. The role of oral contraceptive use.". JAMA 242 (7): 644-8. PMID 221698.
- ↑ STC S.20, 19 Jan 2009.
- ↑ 12.0 12.1 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 923. ISBN 0-7216-0187-1.
- ↑ STC S.19, 19 Jan 2009.
- ↑ SN. 29 May 2009.
- ↑ Katabathina, VS.; Menias, CO.; Shanbhogue, AK.; Jagirdar, J.; Paspulati, RM.; Prasad, SR. (Oct 2011). "Genetics and imaging of hepatocellular adenomas: 2011 update.". Radiographics 31 (6): 1529-43. doi:10.1148/rg.316115527. PMID 21997980.
- ↑ Sasaki, M.; Yoneda, N.; Kitamura, S.; Sato, Y.; Nakanuma, Y. (Oct 2011). "Characterization of hepatocellular adenoma based on the phenotypic classification: The Kanazawa experience.". Hepatol Res 41 (10): 982-8. doi:10.1111/j.1872-034X.2011.00851.x. PMID 21883740.
- ↑ Maylee, H.; Harada, K.; Igarashi, S.; Tohda, G.; Yamamoto, M.; Ren, XS.; Osawa, T.; Hasegawa, Y. et al. (Jun 2012). "Case of telangiectatic/inflammatory hepatocellular adenoma arising in a patient with primary sclerosing cholangitis.". Hepatol Res 42 (6): 611-8. doi:10.1111/j.1872-034X.2011.00962.x. PMID 22568458.
- ↑ Yu, J.; Wang, Y.; Yu, X.; Liang, P.. "Hepatobiliary mucinous cystadenoma and cystadenocarcinoma: report of six cases and review of the literature.". Hepatogastroenterology 57 (99-100): 451-5. PMID 20698207.
- ↑ URL: http://emedicine.medscape.com/article/986802-overview. Accessed on: 29 November 2009.
- ↑ DeBaun MR, Tucker MA (March 1998). "Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry". J. Pediatr. 132 (3 Pt 1): 398–400. PMID 9544889.
- ↑ URL: http://emedicine.medscape.com/article/986802-diagnosis. Accessed on: 11 February 2011.
- ↑ URL: http://emedicine.medscape.com/article/986802-diagnosis. Accessed on: 11 February 2011.
- ↑ URL: http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=cap_foundation%2FcaseOfMonth%2FMar10%2Fmar_2010_cotm_diagnosis.html&_state=maximized&_pageLabel=cntvwr#null. Accessed on: 11 February 2011.
- ↑ 24.0 24.1 Halász, J.; Holczbauer, A.; Páska, C.; Kovács, M.; Benyó, G.; Verebély, T.; Schaff, Z.; Kiss, A. (May 2006). "Claudin-1 and claudin-2 differentiate fetal and embryonal components in human hepatoblastoma.". Hum Pathol 37 (5): 555-61. doi:10.1016/j.humpath.2005.12.015. PMID 16647953.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 588. ISBN 978-0443066573.
- ↑ 26.0 26.1 26.2 26.3 http://emedicine.medscape.com/article/282814-overview
- ↑ 27.0 27.1 27.2 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 924. ISBN 0-7216-0187-1.
- ↑ Leong TY, Leong AS (2005). "Epidemiology and carcinogenesis of hepatocellular carcinoma". HPB (Oxford) 7 (1): 5–15. doi:10.1080/13651820410024021. PMC 2023917. PMID 18333156. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2023917/.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 925. ISBN 0-7216-0187-1.
- ↑ Yusuf MA, Badar F, Meerza F, et al. (2007). "Survival from hepatocellular carcinoma at a cancer hospital in Pakistan". Asian Pac. J. Cancer Prev. 8 (2): 272–4. PMID 17696722.
- ↑ Sharieff S, Burney KA, Ahmad N, Salam A, Siddiqui T (October 2001). "Radiological features of hepatocellular carcinoma in Southern Pakistan". Trop Doct 31 (4): 224–5. PMID 11676064.
- ↑ Adapted from STC (19 Jan 2009).
- ↑ Kojiro, M.; Wanless, IR.; Alves, V.; Badve, S.; Balabaud, C.; Bedossa, P.; Bhathal, P.; Bioulac-Sage, P. et al. (Feb 2009). "Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia.". Hepatology 49 (2): 658-64. doi:10.1002/hep.22709. PMID 19177576. http://onlinelibrary.wiley.com/doi/10.1002/hep.22709/pdf.
- ↑ Adapted from STC (19 Jan 2009).
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 590-1. ISBN 978-0443066573.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 591. ISBN 978-0443066573.
- ↑ Walther, Z.; Jain, D. (2011). "Molecular pathology of hepatic neoplasms: classification and clinical significance.". Patholog Res Int 2011: 403929. doi:10.4061/2011/403929. PMC 3090128. PMID 21559202. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3090128/.
- ↑ 38.0 38.1 38.2 Stipa, F.; Yoon, SS.; Liau, KH.; Fong, Y.; Jarnagin, WR.; D'Angelica, M.; Abou-Alfa, G.; Blumgart, LH. et al. (Mar 2006). "Outcome of patients with fibrolamellar hepatocellular carcinoma.". Cancer 106 (6): 1331-8. doi:10.1002/cncr.21703. PMID 16475212.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 595-6. ISBN 978-0443066573.
- ↑ STC. 6 December 2010.
- ↑ Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. EDMONDSON HA, STEINER PE. Cancer. 1954 May;7(3):462-503. PMID 13160935.
- ↑ 42.0 42.1 Burt, Alastair D.;Portmann, Bernard C.;Ferrell, Linda D. (2006). MacSween's Pathology of the Liver (5th ed.). Churchill Livingstone. pp. 783. ISBN 978-0-443-10012-3.
- ↑ AP. 28 May 2009.
- ↑ Stroescu, C.; Herlea, V.; Dragnea, A.; Popescu, I. (Mar 2006). "The diagnostic value of cytokeratins and carcinoembryonic antigen immunostaining in differentiating hepatocellular carcinomas from intrahepatic cholangiocarcinomas.". J Gastrointestin Liver Dis 15 (1): 9-14. PMID 16680226.
- ↑ Shousha, S.; Gadir, F.; Peston, D.; Bansi, D.; Thillainaygam, AV.; Murray-Lyon, IM. (Oct 2004). "CD10 immunostaining of bile canaliculi in liver biopsies: change of staining pattern with the development of cirrhosis.". Histopathology 45 (4): 335-42. doi:10.1111/j.1365-2559.2004.01927.x. PMID 15469471.
- ↑ Porcell, AI.; De Young, BR.; Proca, DM.; Frankel, WL. (Jul 2000). "Immunohistochemical analysis of hepatocellular and adenocarcinoma in the liver: MOC31 compares favorably with other putative markers.". Mod Pathol 13 (7): 773-8. PMID 10912937.
- ↑ Goodman, ZD. (Feb 2007). "Neoplasms of the liver.". Mod Pathol 20 Suppl 1: S49-60. doi:10.1038/modpathol.3800682. PMID 17486052.
- ↑ URL: http://www.cancer.org/cancer/bileductcancer/detailedguide/bile-duct-cancer-what-is-bile-duct-cancer. Access on: 23 May 2013.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 608. ISBN 978-0443066573.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 926. ISBN 0-7216-0187-1.
- ↑ Park, do H.; Son, HY. (Apr 2008). "Images in clinical medicine. Clonorchis sinensis.". N Engl J Med 358 (16): e18. doi:10.1056/NEJMicm054461. PMID 18420495.
- ↑ de Martel C, Plummer M, Franceschi S (March 2010). "Cholangiocarcinoma: Descriptive epidemiology and risk factors". Gastroenterol Clin Biol. doi:10.1016/j.gcb.2010.01.008. PMID 20202771.
- ↑ Ananthakrishnan AN, Saeian K (April 2007). "Caroli's disease: identification and treatment strategy". Curr Gastroenterol Rep 9 (2): 151–5. PMID 17418061.
- ↑ Nakanishi, Y.; Zen, Y.; Kawakami, H.; Kubota, K.; Itoh, T.; Hirano, S.; Tanaka, E.; Nakanuma, Y. et al. (Jul 2008). "Extrahepatic bile duct carcinoma with extensive intraepithelial spread: a clinicopathological study of 21 cases.". Mod Pathol 21 (7): 807-16. doi:10.1038/modpathol.2008.65. PMID 18425077.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 609. ISBN 978-0443066573.
- ↑ Carrasco, CH; Wallace, S; Charnsangavej, C; Richli, W; Wright, KC; Fanning, T; Gianturco, C (Dec 1985). "Expandable biliary endoprosthesis: an experimental study.". AJR Am J Roentgenol 145 (6): 1279-81. PMID 3877438.
- ↑ STC. 2 October 2009.
- ↑ STC. 6 December 2010.
- ↑ Aishima, S.; Fujita, N.; Mano, Y.; Iguchi, T.; Taketomi, A.; Maehara, Y.; Oda, Y.; Tsuneyoshi, M. (Sep 2010). "p62+ Hyaline inclusions in intrahepatic cholangiocarcinoma associated with viral hepatitis or alcoholic liver disease.". Am J Clin Pathol 134 (3): 457-65. doi:10.1309/AJCP53YVVJCNDZIR. PMID 20716803.
- ↑ Iacobuzio-Donahue, Christine A.; Montgomery, Elizabeth A. (2005). Gastrointestinal and Liver Pathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 609. ISBN 978-0443066573.
- ↑ STC. 6 December 2010.
- ↑ [Evaluation of immunohistochemical markers for differential diagnosis of hepatocellular carcinoma from intrahepatic cholangiocarcinoma] Dong H, Cong WL, Zhu ZZ, Wang B, Xian ZH, Yu H. Zhonghua Zhong Liu Za Zhi. 2008 Sep;30(9):702-5. Chinese. PMID 19173916.
- ↑ Lei JY, Bourne PA, diSant'Agnese PA, Huang J (April 2006). "Cytoplasmic staining of TTF-1 in the differential diagnosis of hepatocellular carcinoma vs cholangiocarcinoma and metastatic carcinoma of the liver". Am. J. Clin. Pathol. 125 (4): 519–25. doi:10.1309/59TN-EFAL-UL5W-J94M. PMID 16627262.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 212. ISBN 978-1416054542.
- ↑ OA. 29 November 2009.
- ↑ The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.