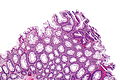
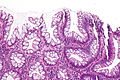
Traditional adenoma
| Traditional adenoma | |
|---|---|
| Diagnosis in short | |
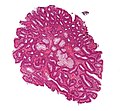
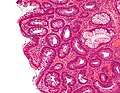
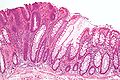
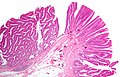
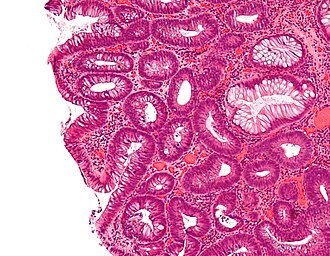 Tubular adenoma. H&E stain. | |
|
| |
| LM | nuclear changes - esp. at the surface (hyperchromatic elongated nuclei or round cleared nuclei), nuclear crowding/pseudostratification, +/- loss of nuclear polarity, loss/decrease of goblet cells, cytoplasmic hyperchromasia |
| Subtypes | tubular adenoma, villous adenoma, tubulovillous adenoma |
| LM DDx | sessile serrated adenoma with dysplasia, reactive changes in other gastrointestinal polyps, invasive adenocarcinoma, adenoma-like adenocarcinoma |
| Site | usually rectum and distal large bowel |
|
| |
| Associated Dx | colorectal adenocarcinoma |
| Syndromes | Familial adenomatous polyposis |
|
| |
| Signs | usually asymptomatic, may present with rectal bleeding |
| Symptoms | uncommon, usually asymptomatic |
| Prevalence | common |
| Blood work | +/-anemia |
| Radiology | polyp |
| Endoscopy | polyp - pedunculated or sessile |
| Prognosis | good |
| Clin. DDx | invasive colorectal adenocarcinoma, benign colorectal mucosa, hyperplastic polyp, other gastrointestinal tract polyps |
| Treatment | usually endoscopic resection (polypectomy) |
| Traditional adenoma | |
|---|---|
| External resources | |
| EHVSC | 10192 (Ki-67 IHC) |
Traditional adenoma refers to a group of pre-cancerous lesions of the gastrointestinal tract.
It includes tubular adenoma, tubulovillous adenoma, and villous adenoma. These are abbreviated TA, TVA and VA respectively.
General
- Most common group of adenomas in gastrointestinal tract.
- Usually arise in the context of an APC mutation.
- Many are seen in the context of familial adenomatous polyposis.
- Size matters: small (<=5 mm) polyps (known as diminutive polyps) are much less likely to be associated with malignancy.[1][2]
Gross
- Polypoid mass or sessile mass.
- Villous adenoma sessile.
Microscopic
- Nuclear changes at the surface of the mucosa - key feature.
- Size and shape or size change:
- Cigar-shaped (elongated) nucleus (usu. length:width > 3:1) with nuclear hyperchromasia (more blue).
- Large round nuclei +/- vesicular appearance (clearing) -- nuclei have white space.
- Nuclear crowding/pseudostratification - important.
- +/-Loss of nuclear polarity (nuclei no longer on basement membrane).
- Size and shape or size change:
- Loss/decrease of goblet cells (common).
- Cytoplasmic hyperchromasia.
Notes:
- Nuclear changes deep to the surface are non-neoplastic if normal appearing mucosa (with small round nuclei) is superficial to it; mucosa that is more blue and atypical deep and less blue without nuclear atypia at the surface is said to be "maturing".
- Classically, adenomatous polyps have "reverse maturation":
- The surface is more hyperchromatic (more blue).
- The base is more mature (more globlet cells, no nuclear changes -- less blue).
- Classically, adenomatous polyps have "reverse maturation":
- Ampullary adenomas often have less prominent pseudostratification and fine chromatin.
DDx:
- Reactive changes due to inflammation.
- Invasive adenocarcinoma.
- Adenoma-like adenocarcinoma.[3]
Images
www:
- TA (flickr.com).
- TA with HGD (flickr.com).
- TA with HGD (daveproject.org).[4]
- TA with HGD (diagnosticpathology.org).[5]
Typing
Subclassified as:[6]
- Tubular adenoma (most common), tubular component >75%.
- Villous adenoma (least common ~= 1% of (traditional) adenomas), villous component >50%.
- Tubulovillous adenoma (uncommon ~5-10% of (traditional) adenomas), villous component >=25% & <=50%.
In other words:
- Tubular T/V >75% / <25%; Tubulovillous T/V <=75%-50% / 25%-<50%; Villous T/V <=50% / >50%.
Note 1:[6]
- Most villous adenomas are sessile, i.e. flat.[7]
- Tubular adenomas tend to be pedunculated, i.e. have a stalk.
- Villous adenomas have a worse prognosis and warrant closer follow-up.
- One needs only to remember the criteria for tubular adenomas and villous adenomas, as tubulovillous adenomas are what is left over.
- Tubular adenomas >75% tubular, Villous adenoma >=50% villous.
- Historically, there were different definitions for tubular adenoma, tubulovillous adenoma, and villous adenomas.[7]
- Health Organization (WHO) criteria: villous adenomas >80% villous architecture.
Note 2:
- There is no formal definition of "villous" architecture.[8]
- Libre Pathology suggests: slender finger-like projections with length-to-width ratio greater than 4.
Note 3:
- The term tubular adenoma is used in different contexts; it should not be confused with Sertoli cell nodule (AKA testicular tubular adenoma).
Grading
Adenomas are usually graded with a two-tier system:[9]
| Feature | Low grade dysplasia (LGD) | High grade dysplasia (HGD) | Importance |
|---|---|---|---|
| Architecture | tubular, minimal focal gland fusion acceptable | any of the following: (gland) cribriforming, glandular budding, intraluminal papillary tufting, sheeting (of epithelium), lamina propria invasion † | key feature |
| Cytology | usu. no features of HGD | any of the following: loss of nuclear stratification, enlarged nuclei, loss of cell polarity, prominent nucleoli, open (clear) chromatin | supportive feature, not sufficient alone for HGD |
Low power colour can be suggestive of HGD:
| Feature | Low grade | High grade |
|---|---|---|
| Colour | light blue | dark blue |
Note:
- † In the colon, unlike other areas of the GI tract, invasive carcinoma is defined by neoplastic cells through the muscularis mucosae. In all other places, e.g. small bowel, invasive carcinoma is defined by neoplastic cells through the basement membrane.
Margins
- Some pathologists believe it is impossible to determine margins in polypectomies.
- Others comment on what they see and then disclaim based on limitations with something like "... margin clear in plane of section."
Haggitt classification
The Haggitt classification is a staging scheme. Surgeons may ask about it 'cause it appears in a widely read surgery textbook and is prognostic. In short:[10][11]
- 0 - high-grade dysplasia. †
- 1 - in submucosa but in head of polyp.
- 2 - neck of polyp.
- 3 - stalk of polyp.
- 4 - submucosa of the bowel wall but above muscularis propria.
It is mostly useless; most polyps do not have a discernible neck or stalk.
Notes:
- † Some refer to this as "intramucosal carcinoma". This term is confusing in the context of colorectal lesions, as cancer is defined by submucosal invasion; it is probably best to avoid using this term.
- Dr. Haggitt is known for GI pathology and his tragic demise.[12] He was shot by a resident that was about to be fired.[13][14]
IHC
- Ki-67 +ve - surface epithelium and upper portion of crypts.[15]
- Absent/minimal staining in cautery artifact.
Sign out
Tubular adenoma - negative for high-grade
A. Colonic Polyp, Transverse Colon, Polypectomy: - Tubular adenoma. - NEGATIVE for high grade dysplasia. B. Colonic Polyp, Transverse Colon, Polypectomy: -Tubular adenoma. -NEGATIVE for high grade dysplasia.
Polyp, Rectum, Polypectomy: - Tubular adenoma. -- NEGATIVE for high-grade dysplasia.
Block letters
POLYP, RECTUM, POLYPECTOMY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
POLYP, SIGMOID COLON, POLYPECTOMY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
POLYP, DESCENDING COLON, POLYPECTOMY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
POLYP, SPLENIC FLEXURE OF COLON, POLYPECTOMY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
POLYP, TRANSVERSE COLON, POLYPECTOMY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
POLYP, HEPATIC FLEXURE OF COLON, POLYPECTOMY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
POLYP, ASCENDING COLON, POLYPECTOMY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
POLYP, CECUM, EXCISION: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
POLYP, LARGE BOWEL AT 30 CM, POLYPECTOMY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
Tubulovillous adenoma - negative for high-grade
Polyp, Sigmoid Colon at 30 cm, Biopsy: - Fragments of tubulovillous adenoma. -- NEGATIVE for high-grade dysplasia. Comment: This biopsy may not be representative of the lesion as a whole. Clinical correlation is required.
Block letters
COLONIC POLYP, SIGMOID COLON, BIOPSY: - TUBULOVILLOUS ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
Villous adenoma - negative for high-grade
COLONIC POLYP, DESCENDING COLON, BIOPSY: - VILLOUS ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA.
Tubular adenoma with focal high-grade dysplasia
POLYP, TRANSVERSE COLON, BIOPSY: - TUBULAR ADENOMA WITH FOCAL HIGH-GRADE DYSPLASIA, MARGINS CLEAR, SEE COMMENT. COMMENT: The case was partially reviewed internally by Dr. Pathology and there is agreement high-grade dysplasia is focally present.
Note:
- "Focal" is something that should prompt review; the management decision often turns on high grade versus low grade.
Tubular adenoma with high-grade dysplasia
POLYP, SIGMOID COLON, BIOPSY: - TUBULAR ADENOMA WITH HIGH-GRADE DYSPLASIA, MARGINS CLEAR.
Invasion cannot be assessed
SIGMOID LESION, 25 CM, BIOPSY: - TUBULAR ADENOMA. - NEGATIVE FOR HIGH-GRADE DYSPLASIA, SEE COMMENT. COMMENT: No stromal desmoplasia is identified. No definite submucosa is present; thus, the presence or absence of definite invasion cannot be assessed.
Biopsy of lesion clinically suspicious for cancer
FLAT LESION, HEPATIC FLEXURE OF COLON, BIOPSY: - TUBULAR ADENOMA. -- NEGATIVE FOR HIGH-GRADE DYSPLASIA. COMMENT: The provided clinical history is noted. The sampled tissue may not be representative of the lesion seen. As it is at least an adenoma, it should be completely excised.
Fragment counting
POLYP, TRANSVERSE COLON, BIOPSY: - TUBULAR ADENOMA (IN 1/3 TISSUE FRAGMENTS). - NEGATIVE FOR HIGH-GRADE DYSPLASIA.
Surgical resection
RECTOSIGMOID, RESECTION: - LARGE TUBULOVILLOUS ADENOMA WITH A SMALL FOCUS OF HIGH-GRADE DYSPLASIA. -- SURGICAL MARGINS NEGATIVE FOR DYSPLASIA. - FOURTEEN LYMPH NODES NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 14 ). - NEGATIVE FOR MALIGNANCY.
Notes
- "Negative for high-grade dysplasia and malignancy" is recommended in the Canadian consensus.[9] The reasoning for the first part is: "with low-grade dysplasia" may lead to over treatment by physicians that are not aware that all (traditional) adenomas have low-grade dysplasia.
- The phrase "negative for [...] malignancy" is also recommended in the Canadian consensus. This is not endorsed by onlinepathology, as one very frequently does not get submucosa. It is like reporting "negative for muscularis propria invasion" on a urinary bladder biopsy without muscularis propria. Further, the guidelines are inconsistent in that they do not advise "negative for dysplasia and malignancy" for SSAs. If there is clinical suspicion of an invasive malignancy, it is useful to comment that no submucosa is present.
Micro
Tubular-tubulovillous interface
The sections shows colorectal-type mucosa with a tubule-forming epithelium that has cellular pseudostratification and enlarged hyperchromatic nuclei, from the crypt base to the luminal aspect (dysplasia).
No cribriforming of glands, epithelial budding or intraluminal papillary tufting is identified. Goblet cells are present in the dysplastic epithelium. Dysplastic nuclei have an ellipsoid-shape and basally stratified.
A small number of rare finger-like epithelial projections (villi) are noted; however these appear to comprise less than 20% of the sampled tissue. It is possible that the villous component is higher or lower due to sampling.
Abbreviated version 1
The sections shows colorectal-type mucosa with a tubule-forming epithelium that has cellular pseudostratification and enlarged hyperchromatic nuclei, from the crypt base to the luminal aspect (dysplasia).
A small number of finger-like epithelial projections (villi) are noted; however, these appear to comprise less than 20% of the sampled tissue.
Abbreviated version 2
A number of finger-like epithelial projections (villi) are noted; however, these appear to comprise less than 25% of the adenomatous tissue seen in section.
More abbreviated
A small number of villous structures are noted; however, these is still in keeping with a tubular adenoma.
Barely villous
The sections shows colorectal-type mucosa with cellular pseudostratification and enlarged hyperchromatic nuclei, from the crypt base to the luminal aspect (dysplasia).
No cribriforming of glands, epithelial budding or intraluminal papillary tufting is identified. Goblet cells are present in the dysplastic epithelium. Dysplastic nuclei have an ellipsoid-shape and basally stratified.
Finger-like epithelial projections (villi) are present and comprise approximately 55% of the dysplastic tissue. The remainder of the lesion has a tubular morphology.
A large morphologically benign lymphoid aggregate is present. No submucosal invasion is identified.
Tubulovillous adenoma
The sections shows colorectal-type mucosa with a tubule-forming and villous-forming epithelium that has cellular pseudostratification and enlarged hyperchromatic nuclei, from the crypt base to the luminal aspect (dysplasia).
No cribriforming of glands, epithelial budding or intraluminal papillary tufting is identified. Goblet cells are rare in the dysplastic epithelium. Dysplastic nuclei have an ellipsoid-shape and basally stratified.
The villous component is over 25% of the lesion but less than 50% of the lesion.
Low-grade versus high-grade
The sections shows colorectal-type mucosa with an epithelium that has cellular pseudostratification and enlarged hyperchromatic nuclei at the the luminal aspect (dysplasia).
The architecture is tubular and villous. The tubular component is predominant.
Focally, the nuclei are round and vesicular, and the glands do not have appreciable numbers of goblet cells. Occasionally, glands are fused and show moderate complexity. No desmoplastic response is evident. Benign fragments of submucosa are present.
Very small adenoma
The sections show colorectal-type mucosa with one dysplastic appearing gland with nuclear hyperchromasia, enlargement and pseudostratification at the surface. It has an abrupt transition to the surrounding normal appearing mucosa. A histomorphologically benign lymphoid aggregate is present.
See also
References
- ↑ Gellad, ZF.; Voils, CI.; Lin, L.; Provenzale, D. (Jun 2013). "Clinical practice variation in the management of diminutive colorectal polyps: results of a national survey of gastroenterologists.". Am J Gastroenterol 108 (6): 873-8. doi:10.1038/ajg.2012.316. PMID 23735908.
- ↑ URL: http://www.internalmedicinenews.com/specialty-focus/gastroenterology/single-article-page/diminutive-polyps-not-likely-to-become-cancerous.html. Accessed on: 5 August 2013.
- ↑ Gonzalez, RS.; Cates, JM.; Washington, MK.; Beauchamp, RD.; Coffey, RJ.; Shi, C. (Jan 2016). "Adenoma-like adenocarcinoma: a subtype of colorectal carcinoma with good prognosis, deceptive appearance on biopsy and frequent KRAS mutation.". Histopathology 68 (2): 183-90. doi:10.1111/his.12725. PMID 25913616.
- ↑ URL: http://daveproject.org/colon-cancer-prevention-flat-lesion-and-endoscopic-mucosal-resection/2011-06-10/. Accessed on: 24 August 2012.
- ↑ Armah, HB.; Krasinskas, AM.; Parwani, AV. (2007). "Tubular adenoma with high-grade dysplasia in the ileal segment 34 years after augmentation ileocystoplasty: report of a first case.". Diagn Pathol 2: 29. doi:10.1186/1746-1596-2-29. PMID 17697327.
- ↑ 6.0 6.1 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 860. ISBN 0-7216-0187-1.
- ↑ 7.0 7.1 URL: http://emedicine.medscape.com/article/170283-overview.
- ↑ R. Riddell. 12 August 2011.
- ↑ 9.0 9.1 Driman, DK.; Marcus, VA.; Hilsden, RJ; Owen, DA (2012). "Pathologic reporting of colorectal polyps: pan-Canadian consensus guidelines". Canadian Journal of Pathology 4 (3): 81-90.
- ↑ URL: http://www.ganfyd.org/index.php?title=Haggitt_classification. Accessed on: 19 March 2011.
- ↑ Haggitt, RC.; Glotzbach, RE.; Soffer, EE.; Wruble, LD. (Aug 1985). "Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy.". Gastroenterology 89 (2): 328-36. PMID 4007423.
- ↑ Rodger C. Haggitt Endowed Chair in Gastroenterology. URL: http://depts.washington.edu/givemed/prof-chair/endowments/rodger-haggitt/. Accessed on: February 2, 2013.
- ↑ Two die in UW medical school shooting. seattlepi.com. URL: http://community.seattletimes.nwsource.com/archive/?date=20000629&slug=4029355. Accessed on: 4 February 2013.
- ↑ URL: http://www.washington.edu/alumni/columns/sept00/choices.html. Accessed on: 4 February 2013.
- ↑ Groisman, GM.; Amar, M.; Meir, A. (Jul 2007). "Utility of MIB-1 (Ki-67) in evaluating diminutive colorectal polyps with cautery artifact.". Arch Pathol Lab Med 131 (7): 1089-93. doi:10.1043/1543-2165(2007)131[1089:UOMKIE]2.0.CO;2. PMID 17616996.