Difference between revisions of "Liver neoplasms"
(→Cholangiocarcinoma: +images) |
(→Hematopoietic tumors: added a case) |
||
| (112 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
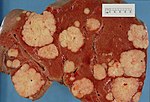
This article examines '''liver neoplasms''' and '''pre-malignant lesions of the liver'''. In North America, most malignant liver lesions are metastases. | [[Image:Secondary tumor deposits in the liver from a primary cancer of the pancreas.jpg|thumb|right|300px|Liver metastases at [[gross pathology|gross]].]] | ||
This article examines '''liver neoplasms''' and '''pre-malignant lesions of the liver'''. In North America, most malignant liver lesions are [[liver metastasis|metastases]]. | |||
This article focuses on primary malignancies of the liver, neoplastic liver lesions, and biliary malignancies. | This article focuses on primary malignancies of the liver, neoplastic liver lesions, and biliary malignancies. | ||
It only briefly discusses metastatic lesions. An introduction to ''liver pathology'' is in the ''[[liver]]'' article. Medical liver disease is dealt with in the ''[[medical liver disease]]'' article. | It only briefly discusses metastatic lesions. An introduction to ''liver pathology'' is in the ''[[liver]]'' article. Medical liver disease is dealt with in the ''[[medical liver disease]]'' article. | ||
=Overview= | |||
===Dysplasic lesions of the liver=== | ===Dysplasic lesions of the liver=== | ||
Types:<ref>STC. S.30-37, 19 Jan 2009.</ref> | Types:<ref>STC. S.30-37, 19 Jan 2009.</ref> | ||
*"Large cell dysplasia" (AKA ''large cell change'') - not considered a precursor for HCC, not considered a dysplasia.<ref name=pmid16982109>{{Cite journal | last1 = Park | first1 = YN. | last2 = Roncalli | first2 = M. | title = Large liver cell dysplasia: a controversial entity. | journal = J Hepatol | volume = 45 | issue = 5 | pages = 734-43 | month = Nov | year = 2006 | doi = 10.1016/j.jhep.2006.08.002 | PMID = 16982109 }}</ref> | *"Large cell dysplasia" (AKA ''large cell change'') - not considered a precursor for HCC, not considered a dysplasia.<ref name=pmid16982109>{{Cite journal | last1 = Park | first1 = YN. | last2 = Roncalli | first2 = M. | title = Large liver cell dysplasia: a controversial entity. | journal = J Hepatol | volume = 45 | issue = 5 | pages = 734-43 | month = Nov | year = 2006 | doi = 10.1016/j.jhep.2006.08.002 | PMID = 16982109 }}</ref> | ||
*Small cell dysplasia. | *[[Small liver cell dysplasia]] ([[AKA]] small cell dysplasia). | ||
*Low grade dysplasia. | *Low grade dysplasia. | ||
*High grade dysplasia. | *High grade dysplasia. | ||
===Neoplastic lesions=== | ===Neoplastic lesions=== | ||
*Hepatic adenoma. | *[[Hepatic adenoma]]. | ||
===Malignant lesions of the liver=== | ===Malignant lesions of the liver=== | ||
*Hepatocellular carcinoma (HCC) - most common malignant liver primary in adults. | *[[Hepatocellular carcinoma]] (HCC) - most common malignant liver primary in adults. | ||
*Hepatoblastoma - malignant liver primary in children. | *[[Hepatoblastoma]] - malignant liver primary in children. | ||
*Intrahepatic cholangiocarcinoma (ICC).<ref name=pmid19212669>{{Cite journal | last1 = Shirakawa | first1 = H. | last2 = Kuronuma | first2 = T. | last3 = Nishimura | first3 = Y. | last4 = Hasebe | first4 = T. | last5 = Nakano | first5 = M. | last6 = Gotohda | first6 = N. | last7 = Takahashi | first7 = S. | last8 = Nakagohri | first8 = T. | last9 = Konishi | first9 = M. | title = Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. | journal = Int J Oncol | volume = 34 | issue = 3 | pages = 649-56 | month = Mar | year = 2009 | doi = | PMID = 19212669 | url = http://www.spandidos-publications.com/serveFile/ijo_34_3_649_PDF.pdf?type=article&article_id=ijo_34_3_649&item=PDF}}</ref> | *Intrahepatic [[cholangiocarcinoma]] (ICC).<ref name=pmid19212669>{{Cite journal | last1 = Shirakawa | first1 = H. | last2 = Kuronuma | first2 = T. | last3 = Nishimura | first3 = Y. | last4 = Hasebe | first4 = T. | last5 = Nakano | first5 = M. | last6 = Gotohda | first6 = N. | last7 = Takahashi | first7 = S. | last8 = Nakagohri | first8 = T. | last9 = Konishi | first9 = M. | title = Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. | journal = Int J Oncol | volume = 34 | issue = 3 | pages = 649-56 | month = Mar | year = 2009 | doi = | PMID = 19212669 | url = http://www.spandidos-publications.com/serveFile/ijo_34_3_649_PDF.pdf?type=article&article_id=ijo_34_3_649&item=PDF}}</ref> | ||
*Combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma (CHC). | *Combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma (CHC). | ||
===Lesions that arise in a non-cirrhotic liver=== | |||
Hepatocellular: | |||
*[[Hepatic adenoma]]. | |||
*[[Fibrolamellar hepatocellular carcinoma]]. | |||
*[[Focal nodular hyperplasia]]. | |||
Other: | |||
*[[Metastasis]]. | |||
*[[Cholangiocarcinoma]]. | |||
*[[Liver hemangioma]]. | |||
===Tabular comparison=== | ===Tabular comparison=== | ||
====Precursors==== | |||
Features of HCC & its precursors - generated from DCHH<ref name=Ref_DCHH170-1>{{Ref DCHH|170-1}}</ref> and STC: | Features of HCC & its precursors - generated from DCHH<ref name=Ref_DCHH170-1>{{Ref DCHH|170-1}}</ref> and STC: | ||
{| class="wikitable" | {| class="wikitable" | ||
| '''Features''' || ''' | | '''Features''' || '''SLCD''' || '''Low-grade dysplasia''' || '''High-grade dysplasia''' || '''HCC''' | ||
|- | |- | ||
| Plate thickness || <3 cells || <=2 cells || <=3 cells, usu. >2 cells || '''>3 cells''' | | Plate thickness || <3 cells || <=2 cells || <=3 cells, usu. >2 cells || '''>3 cells''' | ||
| Line 41: | Line 53: | ||
|} | |} | ||
Abbreviations: | Abbreviations: | ||
* | *SLCD = small liver cell dysplasia. | ||
Notes: | Notes: | ||
*Large cell dysplasia: | *Large cell dysplasia: | ||
**Cell size ~ 2x normal, [[NC ratio]] ~ normal. | **Cell size ~ 2x normal, [[NC ratio]] ~ normal. | ||
* | *SLCD: | ||
**Cell size ~ 1/2x normal, NC ratio - increased. | **Cell size ~ 1/2x normal, NC ratio - increased. | ||
==Small cell dysplasia== | ====Hepatic tumours==== | ||
Benign: | |||
{| class="wikitable sortable" | |||
! Entity | |||
! Gross | |||
! Microscopic | |||
! IHC/stains | |||
! Other | |||
! Images | |||
|- | |||
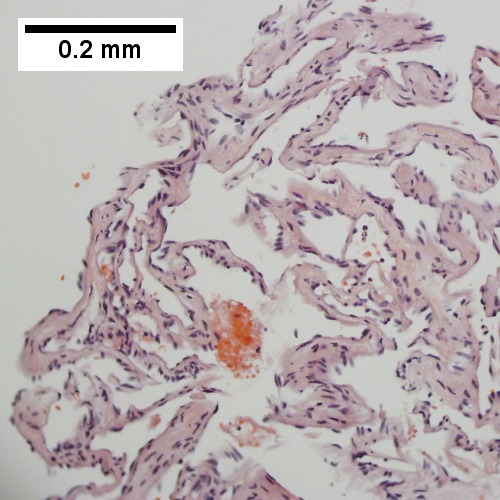
| [[Hepatic hemangioma]] | |||
| similar to normal liver parenchyma, red (hemorrhagic), well-circumscribed | |||
| spaces lined by benign endothelial cells | |||
| CD31+ (???) | |||
| - | |||
| [http://radiographics.rsna.org/content/24/6/1719/F8.expansion.html gross (rsna.org)] | |||
|- | |||
| [[Focal nodular hyperplasia]] | |||
| central scar, large vessels, usu. well-circumscribed | |||
| large arteries, unpaired arteries, bile duct proliferation | |||
| | |||
| usu. diagnosed by imaging | |||
| [http://radiographics.rsna.org/content/24/1/3/F1.expansion.html gross (rsna.org)] | |||
|- | |||
| [[Hepatocellular adenoma]] | |||
| subcapsular, well-circumscribed | |||
| loss of portal tracts, nuclear glycogenation | |||
| reticulin - liver plate thickness <= 3 | |||
| background not cirrhotic, assoc. [[OCP]] | |||
| [http://www.mda-sy.com/up//uploads/images/mda-sy-d768fd265c.jpg gross (mda-sy.com)<ref>URL: [http://www.mda-sy.com/vb/showthread.php?p=5083&langid=1 http://www.mda-sy.com/vb/showthread.php?p=5083&langid=1]. Accessed on: 16 February 2012.</ref>] | |||
|} | |||
Malignant: | |||
{| class="wikitable sortable" | |||
! Entity | |||
! Gross | |||
! Microscopic | |||
! IHC/stains | |||
! Other | |||
! Images | |||
|- | |||
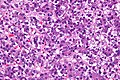
| [[Liver metastasis]] | |||
| multiple, white lesions | |||
| variable, usu. tubular (glandular) with pseudostratified hyperchromatic nuclei | |||
| CK7-, [[CK20]]+ (colorectal), HepPar-1-, [[CK19]]- | |||
| [[colorectal carcinoma]] most common | |||
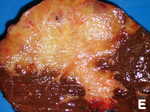
| [[Image:Secondary_tumor_deposits_in_the_liver_from_a_primary_cancer_of_the_pancreas.jpg | thumb| center| 150px| Metastases. (WC)]] | |||
|- | |||
| [[Hepatocellular carcinoma]] | |||
| poorly circumscribed, +/-necrosis, +/-hemorrhage | |||
| loss of portal tracts, unpaired arteries, +/-nuclear atypia | |||
| reticulin - liver plate thickness > 3 | |||
| background often cirrhotic | |||
| [[Image:Hepatocellular_carcinoma_1.jpg |thumb|center|150px| HCC. (WC/Uthman)]] | |||
|- | |||
| [[Cholangiocarcinoma]] | |||
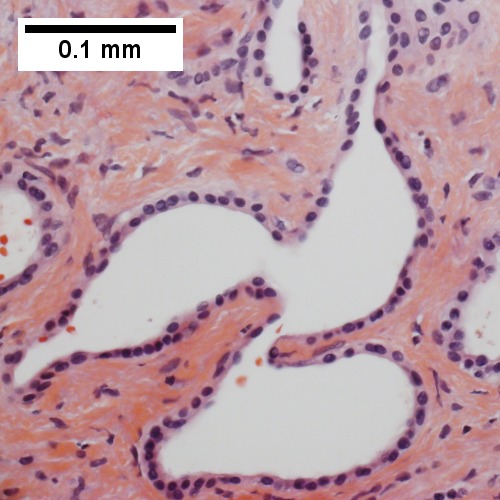
| cauliflower-like outline, white, classically solitary, no cirrhosis | |||
| tubular architecture and mild [[nuclear atypia]] (adenocarcinoma), [[desmoplastic stroma]] | |||
| CK7+, CK19+ | |||
| background usu. not cirrhotic | |||
| [[Image:Cholangiocarcinoma.png |thumb|center|150px| Cholangiocarcinoma. (WC)]] | |||
|} | |||
=Dysplasia of the liver= | |||
==Small liver cell dysplasia== | |||
*Abbreviated ''SLCD''. | |||
*[[AKA]] ''small cell dysplasia''. | |||
===General=== | ===General=== | ||
*Considered a precursor to HCC. | *Considered a precursor to [[HCC]]. | ||
**Frequently found in livers with HCC - when compared to livers without HCC.<ref name=pmid9401407>{{Cite journal | last1 = Szczepański | first1 = W. | title = Liver cell dysplasia in liver cirrhosis and hepatocellular carcinoma. | journal = Pol J Pathol | volume = 48 | issue = 3 | pages = 147-57 | month = | year = 1997 | doi = | PMID = 9401407 }}</ref> | |||
=== | ===Microscopic=== | ||
Features:<ref>STC S.32, 19 Jan 2009.</ref> | Features:<ref>STC S.32, 19 Jan 2009.</ref> | ||
*Cells similar in size to normal hepatocytes. | *Cells similar in size to normal hepatocytes. | ||
**Name derived from the fact that there is also an entity that was called ''large cell dysplasia'' (AKA ''large cell change''). | **Name derived from the fact that there is also an entity that was called ''large cell dysplasia'' (AKA ''large liver cell dysplasia'',<ref name=pmid9401407>{{Cite journal | last1 = Szczepański | first1 = W. | title = Liver cell dysplasia in liver cirrhosis and hepatocellular carcinoma. | journal = Pol J Pathol | volume = 48 | issue = 3 | pages = 147-57 | month = | year = 1997 | doi = | PMID = 9401407 }}</ref> and ''large cell change''). | ||
*Increased [[NC ratio]] - "more blue". | *Increased [[NC ratio]] - "more blue". | ||
*Mild nuclear and cytoplasmic hyperchromatism. | *Mild nuclear and cytoplasmic hyperchromatism. | ||
| Line 67: | Line 146: | ||
*[http://www.medscape.com/viewarticle/515219_3 Low resolution micrograph of SCD (medscape.com)]. | *[http://www.medscape.com/viewarticle/515219_3 Low resolution micrograph of SCD (medscape.com)]. | ||
==Low grade dysplasia== | ==Low-grade hepatocellular dysplasia== | ||
=== | *Generally referred to as ''low-grade dysplasia'' as the context is usually clear. | ||
===Microscopic=== | |||
*Uniform cells - "noticeably different from normal".<ref>STC - 19 Jan 2009. (???)</ref> | *Uniform cells - "noticeably different from normal".<ref>STC - 19 Jan 2009. (???)</ref> | ||
**Changes in nuclear size, irregular nuclear contour and/or changes in cytoplasm staining. | **Changes in nuclear size, irregular nuclear contour and/or changes in cytoplasm staining. | ||
| Line 80: | Line 160: | ||
*[[Nodular regenerative hyperplasia]] - lacks: compressed rim of cells, central portal tract.<ref name=Ref_DCHH170-1>{{Ref DCHH|170-1}}</ref> | *[[Nodular regenerative hyperplasia]] - lacks: compressed rim of cells, central portal tract.<ref name=Ref_DCHH170-1>{{Ref DCHH|170-1}}</ref> | ||
==High grade dysplasia== | ==High-grade hepatocellular dysplasia== | ||
*"Bader" version of low grade dyplasia. | *Generally referred to as ''high-grade dysplasia'' as the context is usually clear. | ||
===General=== | |||
*"Bader" version of ''[[Low-grade hepatocellular dysplasia|low-grade dyplasia]]''. | |||
===Microscopic=== | |||
Features - in addition to those of low grade dysplasia:<ref name=Ref_DCHH170-1>{{Ref DCHH|170-1}}</ref> | Features - in addition to those of low grade dysplasia:<ref name=Ref_DCHH170-1>{{Ref DCHH|170-1}}</ref> | ||
*Liver plate >2 cells thick. | *Liver plate >2 cells thick. | ||
| Line 88: | Line 171: | ||
*Basophilic cytoplasm. | *Basophilic cytoplasm. | ||
DDx: | |||
*[[Low-grade hepatocellular dysplasia]]. | |||
*[[Hepatocellular carcinoma]]. | |||
Image: | |||
*[http://radiographics.rsna.org/content/22/5/1023.figures-only Series of liver micrographs including high grade dysplasia (radiographics.rsna.org)]. | *[http://radiographics.rsna.org/content/22/5/1023.figures-only Series of liver micrographs including high grade dysplasia (radiographics.rsna.org)]. | ||
== | =Benign hepatic neoplasms= | ||
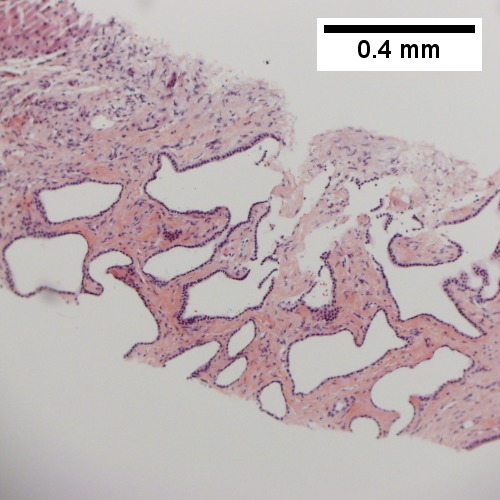
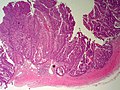
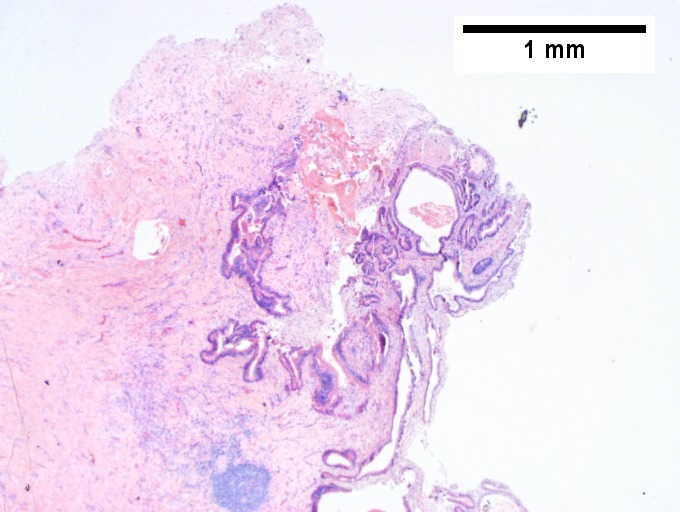
==Bile duct hamartoma== | |||
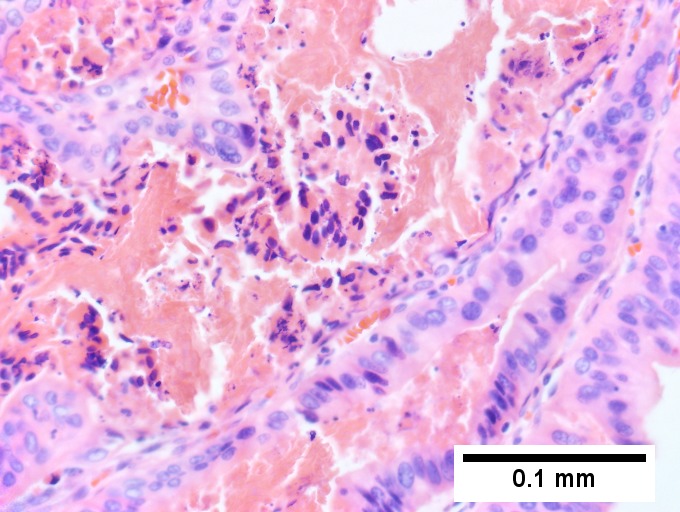
A. [[File:1 BDH 1 680x512px.tif|Trichrome shows fibrous spaces with dilated ducts (20X).]] | |||
B. [[File:2 BDH 1 680x512px.tif|Bizarre, ramifying tubules with dilatations (100X).]] | |||
<br> | |||
C. [[File:3 BDH 1 680x512px.tif|Bland epithelial linings (400X).]] | |||
D. [[File:4 BDH 1 680x512px.tif|Surrounding tract with tortuous bile ducts & inflammation, likely secondary to hamartomas (200X).]] | |||
Bile duct hamartomas. A. Trichrome shows fibrous spaces with dilated ducts. B. Bizarre, ramifying tubules with dilatations. C. Bland epithelial linings. D. Surrounding tract with tortuous bile ducts & inflammation, likely secondary to hamartomas. | |||
==Bile duct adenoma== | |||
:''Should '''not''' be confused with [[bile duct hamartoma]].'' | |||
{{Main|Bile duct adenoma}} | |||
==Hepatic adenoma== | ==Hepatic adenoma== | ||
*[[AKA]] ''hepatocellular adenoma'', abbreviated ''HCA''. | *[[AKA]] ''hepatocellular adenoma'', abbreviated ''HCA''. | ||
{{Main|Hepatic adenoma}} | |||
==Hepatobiliary mucinous cystadenoma== | |||
*[[AKA]] ''biliary cystadenoma''. | |||
===General=== | ===General=== | ||
* | *Benign neoplasm. | ||
**May transform into a malignancy.<ref name=pmid20698207>{{Cite journal | last1 = Yu | first1 = J. | last2 = Wang | first2 = Y. | last3 = Yu | first3 = X. | last4 = Liang | first4 = P. | title = Hepatobiliary mucinous cystadenoma and cystadenocarcinoma: report of six cases and review of the literature. | journal = Hepatogastroenterology | volume = 57 | issue = 99-100 | pages = 451-5 | month = | year = | doi = | PMID = 20698207 }}</ref> | |||
**May | |||
=== | |||
===Microscopic=== | ===Microscopic=== | ||
Features: | Features: | ||
* | *Cystic spaces lined by a mucinous epithelium (simple columnar epithelium with a clear cytoplasm). | ||
* | *Surrounding dense ovarian like stroma. | ||
DDX: | |||
[[Biliary Intraductal Papillary Neoplasm]] | |||
* | *no surrounding ovarian stroma | ||
* | *Intraductal - connects with the biliary tree lumen. | ||
Note: | |||
*[ | *Similar to [[pancreatic mucinous cystadenoma]]. | ||
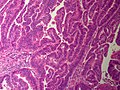
==Cavernous hemangioma== | |||
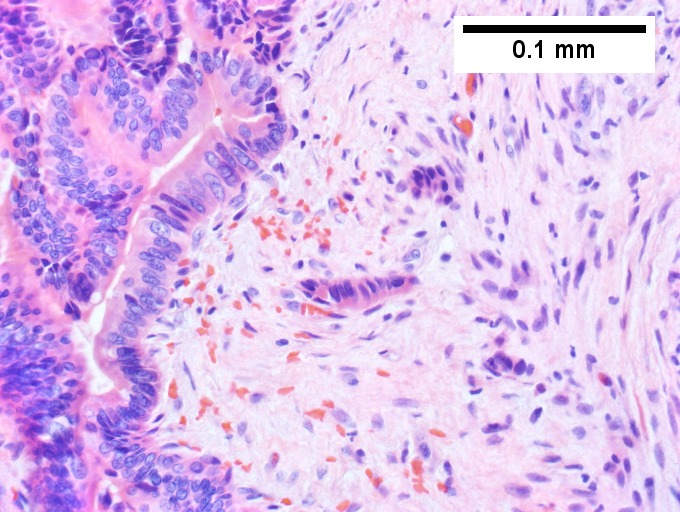
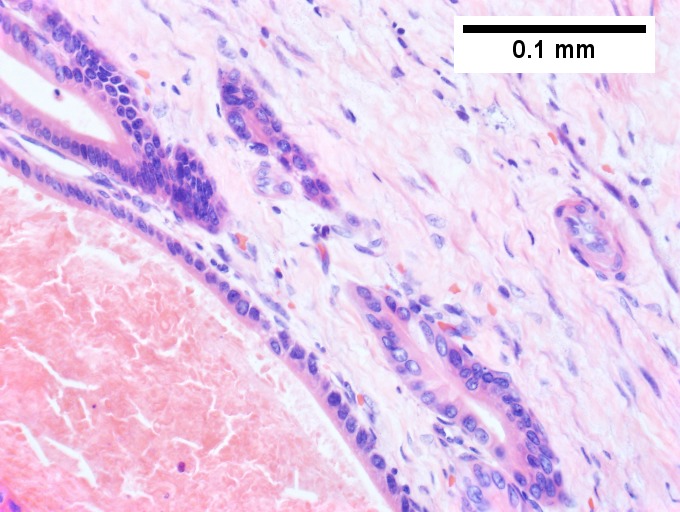
A. [[File:1 CAV 1 680x512px.tif|Fibrous foci with increased spaces, hepatocyte focus with nonspecific fibrotic bridge (40X).]] | |||
B. [[File:2 CAV 1 680x512px.tif|Cavernous hemangioma with flat, non-atypical endothelium (200X).]] | |||
<br> | |||
C. [[File:3 CAV 1 680x512px.tif|Tortuous bile ducts/ductules, not to be considered generalized in presence of mass (200X).]] | |||
D. [[File:4 CAV 1 680x512px.tif|Tortuous bile ductsductules, not to be considered generalized in presence of mass (200X).]] | |||
Cavernous hemangioma. A. Fibrous foci with increased spaces, hepatocyte focus with nonspecific fibrotic bridge. B. Cavernous hemangioma with flat, non-atypical endothelium. C. Tortuous bile ductules, not to be considered generalized in presence of mass. D. Tortuous bile ducts, not to be considered generalized in presence of mass. | |||
== | =Malignant hepatic neoplasms= | ||
In North America, the most common malignant liver tumour is [[liver metastasis|metastases]]. | |||
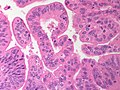
==Hepatoblastoma== | ==Hepatoblastoma== | ||
| Line 154: | Line 238: | ||
Associations: | Associations: | ||
*Beckwith-Wiedemann syndrome.<ref name=pmid9544889>{{cite journal |author=DeBaun MR, Tucker MA |title=Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry |journal=J. Pediatr. |volume=132 |issue=3 Pt 1 |pages=398–400 |year=1998 |month=March |pmid=9544889 |doi= |url=}}</ref> | *[[Beckwith-Wiedemann syndrome]].<ref name=pmid9544889>{{cite journal |author=DeBaun MR, Tucker MA |title=Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry |journal=J. Pediatr. |volume=132 |issue=3 Pt 1 |pages=398–400 |year=1998 |month=March |pmid=9544889 |doi= |url=}}</ref> | ||
*[[Familial adenomatous polyposis]]. | *[[Familial adenomatous polyposis]]. | ||
Clinical: | Clinical: | ||
*Usually present with hepatomegaly. | |||
*High AFP.<ref>URL: [http://emedicine.medscape.com/article/986802-diagnosis http://emedicine.medscape.com/article/986802-diagnosis]. Accessed on: 11 February 2011.</ref> | *High AFP.<ref>URL: [http://emedicine.medscape.com/article/986802-diagnosis http://emedicine.medscape.com/article/986802-diagnosis]. Accessed on: 11 February 2011.</ref> | ||
| Line 167: | Line 252: | ||
**Immature fibrous tissue, osteoid or cartilage. | **Immature fibrous tissue, osteoid or cartilage. | ||
DDx: | |||
*[ | *[[Small round cell tumours]]. | ||
**[[Neuroblastoma]]. | |||
*[[Teratoma]]. | |||
*[[Hepatocellular carcinoma]] - separated based on histomorphology alone. | |||
====Images==== | |||
<gallery> | |||
Image:Hepatoblastoma_-_2_-_very_high_mag.jpg | Hepatoblastoma - very high mag. (WC/Nephron) | |||
Image:Hepatoblastoma_-_high_mag.jpg | Hepatoblastoma - high mag. (WC/Nephron) | |||
</gallery> | |||
====Subtypes==== | ====Subtypes==== | ||
| Line 178: | Line 271: | ||
**# Macrotrabecular pattern. | **# Macrotrabecular pattern. | ||
**#* May mimic [[hepatocellular carcinoma]] histologically.<ref>URL: [http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=cap_foundation%2FcaseOfMonth%2FMar10%2Fmar_2010_cotm_diagnosis.html&_state=maximized&_pageLabel=cntvwr#null http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=cap_foundation%2FcaseOfMonth%2FMar10%2Fmar_2010_cotm_diagnosis.html&_state=maximized&_pageLabel=cntvwr#null]. Accessed on: 11 February 2011.</ref> | **#* May mimic [[hepatocellular carcinoma]] histologically.<ref>URL: [http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=cap_foundation%2FcaseOfMonth%2FMar10%2Fmar_2010_cotm_diagnosis.html&_state=maximized&_pageLabel=cntvwr#null http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=cap_foundation%2FcaseOfMonth%2FMar10%2Fmar_2010_cotm_diagnosis.html&_state=maximized&_pageLabel=cntvwr#null]. Accessed on: 11 February 2011.</ref> | ||
**# Small cell undifferentiated pattern | **# Small cell undifferentiated pattern. | ||
**#* Poor prognosis. | |||
** Mixed epithelial and mesenchymal type: | ** Mixed epithelial and mesenchymal type: | ||
**# With teratoid features. | **# With teratoid features. | ||
**# Without teratoid features. | **# Without teratoid features. | ||
===IHC=== | |||
*Alpha-fetoprotein +ve. | |||
*Hepatocyte specific antigen +ve esp. in fetal component.<ref name=pmid16647953>{{Cite journal | last1 = Halász | first1 = J. | last2 = Holczbauer | first2 = A. | last3 = Páska | first3 = C. | last4 = Kovács | first4 = M. | last5 = Benyó | first5 = G. | last6 = Verebély | first6 = T. | last7 = Schaff | first7 = Z. | last8 = Kiss | first8 = A. | title = Claudin-1 and claudin-2 differentiate fetal and embryonal components in human hepatoblastoma. | journal = Hum Pathol | volume = 37 | issue = 5 | pages = 555-61 | month = May | year = 2006 | doi = 10.1016/j.humpath.2005.12.015 | PMID = 16647953 }}</ref> | |||
*Beta-catenin +ve (cytoplasmic and nuclear).<ref name=pmid16647953/> | |||
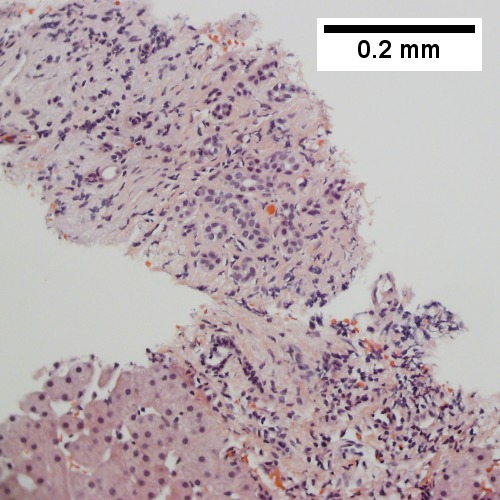
==Hepatocellular carcinoma== | ==Hepatocellular carcinoma== | ||
*Abbreviated ''HCC''. | |||
{{Main|Hepatocellular carcinoma}} | |||
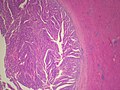
==Biliary Intraductal Papillary Neoplasm<ref>{{Cite journal | Masayuki Ohtsuka, Hiroaki Shimizu, Atsushi Kato, et al., “Intraductal Papillary Neoplasms of the Bile Duct,” International Journal of Hepatology, vol. 2014, Article ID 459091, 10 pages, 2014. doi:10.1155/2014/459091}}</ref>== | |||
===General=== | ===General=== | ||
* | *Rare | ||
*Highest incidence in Far Eastern countries | |||
*Association with hepatolithiasis and clonorchiasis | |||
*Between 50 and 70 years of age | |||
*Slight male predominance | |||
*Intermittent abdominal pain | |||
*Acute cholangitis | |||
*Jaundice | |||
*Biliary counterpart of [[pancreatic intraductal papillary mucinous neoplasm]] | |||
* | *Biliary counterpart of [[intracholecystic papillary neoplasm]] (gall bladder) | ||
* | *Construct consumes some cases of biliary cystadenoma/cystadenocarcinoma, biliary papilloma/papillomatosis, intraductal growth type of cholangiocarcinoma and papillary carcinoma of the extrahepatic bile duct. | ||
==== | ===Radiology=== | ||
*Bile duct dilatation | |||
*Intraductal masses | |||
* | |||
* | |||
===Gross=== | ===Gross=== | ||
*Singular, or occasionally multiple, polypoid masses extending into the lumen of a dilated bile duct | |||
* | *Or multilocular well-defined cystic mass containing mucinous fluid | ||
** | *Granular or papillary mucosa | ||
* | *Communication with bile duct may be difficult to confirm | ||
===Microscopic=== | ===Microscopic=== | ||
*Papillary or villous growth within the lumen of an intra or extrahepatic bile duct | |||
* | *Papillary fronds with fine vascular cores | ||
** | *Epithelium types | ||
** | **Pancreatobiliary | ||
** | **Intestinal - marked mucin secretion | ||
** | **Gastric | ||
** | **Oncocytic types | ||
*Dysplasia | |||
**High or low grade | |||
**Marked variation in histologic grade between different regions of individual tumors | |||
*Common association with invasive cholangiocarcinoma | |||
* | **Tubular adenocarcinoma | ||
** | **Mucinous (colloid) carcinoma (often in association with the intestinal type). | ||
** | |||
====DDX==== | |||
*[[Biliary Mucinous Cystic Neoplasm]] | |||
*[[ | ***Epithelium is surrounded by a distinct ovarian-like stroma. | ||
* | |||
* | |||
====Photos==== | |||
<gallery> | |||
Image:BileDuct IntraductalPapillaryNeoplasm LP CTR.jpg|Bile Ducts - Intraductal Papillary Neoplasm - Low power (SKB) | |||
Image:BileDuct IntraductalPapillaryNeoplasm MP CTR.jpg|Bile Ducts - Intraductal Papillary Neoplasm - Medium power (SKB) | |||
Image:BileDuct IntraductalPapillaryNeoplasm HP CTR.jpg|Bile Ducts - Intraductal Papillary Neoplasm - High power (SKB) | |||
Image:BileDucts IntraductalPapillaryNeoplasm NonMucinousType LP PA.jpg|Bile Ducts - Intraductal Papillary Neoplasm - Low power (SKB) | |||
Image:BileDucts IntraductalPapillaryNeoplasm OncocyticType HP PA.jpg|Bile Ducts - Intraductal Papillary Neoplasm - High power (SKB) | |||
Image:BileDucts IntraductalPapillaryNeoplasm NonMucinousType HP2 PA.jpg|Bile Ducts - Intraductal Papillary Neoplasm - High power (SKB) | |||
</gallery> | |||
A. [[File:1 papillary cbd aca 1 680x512px.tif| Intraductal papillary neoplasm of common bile duct with associated invasive carcinoma.]] | |||
B. [[File:2 papillary cbd aca 1 680x512px.tif| Intraductal papillary neoplasm of common bile duct with associated invasive carcinoma.]] | |||
<br> | |||
C. [[File:3 papillary cbd aca 1 680x512px.tif| Intraductal papillary neoplasm of common bile duct with associated invasive carcinoma.]] | |||
D. [[File:4 papillary cbd aca 1 680x512px.tif| Intraductal papillary neoplasm of common bile duct with associated invasive carcinoma.]] | |||
<br> | |||
E. [[File:5 papillary cbd aca 1 680x512px.tif| Intraductal papillary neoplasm of common bile duct with associated invasive carcinoma.]] | |||
F. [[File:6 papillary cbd aca 1 680x512px.tif| Intraductal papillary neoplasm of common bile duct with associated invasive carcinoma.]] | |||
Intraductal papillary neoplasm of common bile duct with associated invasive carcinoma. A. The papillary tumor comprises mostly variably dilated acini. B. Tumor also shows areas of micropapillae. C. Some areas within the non-invasive tumor showed necrosis, with the black pyknotic nuclei amid red debris. D. Definite invasion was established low power by glands headed in perpendicular directions. E. Embedded in fibroblastic response are non-acinar walls and isolated epithelial groups. F. Also embedded in fibroblastic response are flat glands with nuclei showing loss of polarity (lack of respect for lateral intercellular borders shown by variable orientation to base of gland). | |||
Notes - | |||
*Reflect on the known marked variation in histologic grade between different regions of individual tumors when rendering an opinion on a small biopsy specimen. | |||
*Consider the possibility of an invasive component and submit tissue generously. | |||
* | |||
* | |||
See also: | |||
PubCan [http://www.pubcan.org/printicdotopo.php?id=5755] | |||
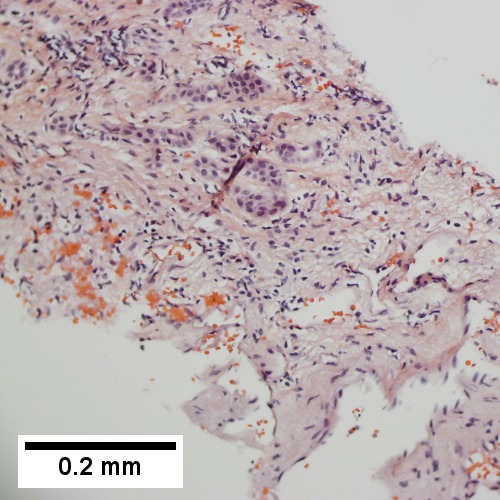
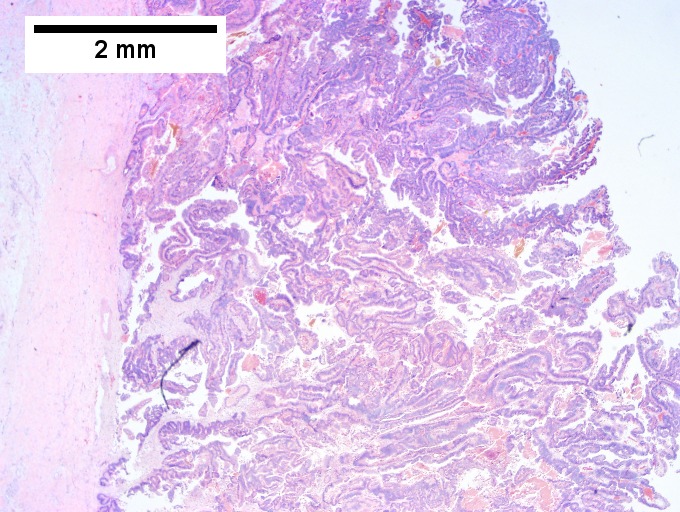
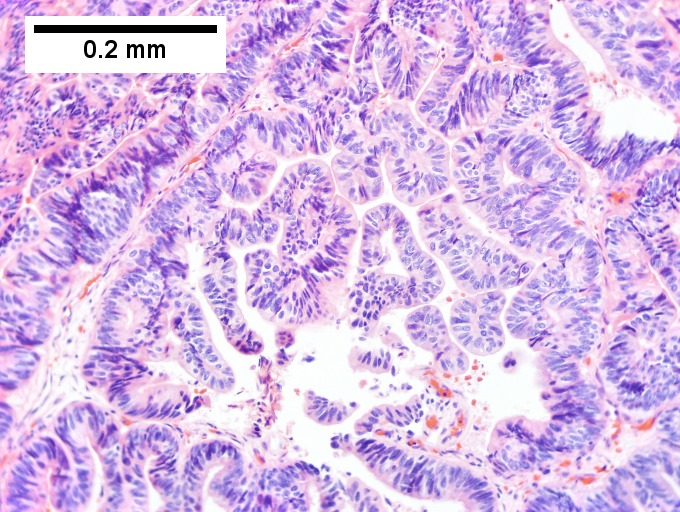
==Cholangiocarcinoma== | ==Cholangiocarcinoma== | ||
*[[AKA]] ''bile duct carcinoma''.<ref>URL: [http://www.cancer.org/cancer/bileductcancer/detailedguide/bile-duct-cancer-what-is-bile-duct-cancer http://www.cancer.org/cancer/bileductcancer/detailedguide/bile-duct-cancer-what-is-bile-duct-cancer]. Access on: 23 May 2013.</ref> | |||
{{Main|Cholangiocarcinoma}} | |||
* | |||
==Hepatic angiosarcoma== | |||
== | |||
{{Main|Angiosarcoma}} | {{Main|Angiosarcoma}} | ||
*[[AKA]] ''angiosarcoma of the liver''. | |||
===General=== | ===General=== | ||
*Liver angiosarcomas are associated with vinyl chloride exposure.<ref name=Ref_PCPBoD8_212>{{Ref PCPBoD8|212}}</ref> | *Liver angiosarcomas are associated with vinyl chloride exposure.<ref name=Ref_PCPBoD8_212>{{Ref PCPBoD8|212}}</ref> | ||
| Line 368: | Line 374: | ||
*Atypical endothelial cells - may be subtle. | *Atypical endothelial cells - may be subtle. | ||
== | ==Hepatic metastasis== | ||
* | {{Main|Liver metastasis}} | ||
*[[AKA]] ''liver metastases''. | |||
* | *[[AKA]] ''metastatic liver disease''. | ||
==Hematopoietic tumors== | |||
A [[File:1 MM 1 Covenant 680x512px.tif|One liver core was normal (Row 1 Left 40X).]] | |||
<br> | <br> | ||
B [[File:2 MM 1 Covenant 680x512px.tif|A triad with a proliferated bile ductule, otherwise normal (Row 1 Right 400X).]] | |||
<br> | |||
C [[File:3 MM 1 Covenant 680x512px.tif|The other core showed a mass of tumor mashed against normal liver (Row 2 Left 40X).]] | |||
<br> | |||
D [[File:4 MM 1 Covenant 680x512px.tif|Tumor cells showed round to ovoid nuclei without pattern and with grey cytoplasm that proved to be CD138 positive (Row 2 Right 400X).]] | |||
<br> | |||
Plasmacytoma appearing as a tumor mass. A. One liver core was normal. B. A triad with a proliferated bile ductule, otherwise normal. C. The other core showed a mass of tumor mashed against normal liver. D. Tumor cells showed round to ovoid nuclei without pattern and with grey cytoplasm that proved to be CD138 positive. | |||
A. [[File:1 B cell lym liver 1 680x512px.tif|Apparent inflamed fibrous tract with lobular inflammatory collections in adjacent liver (Row 1 Left 40X).]] | |||
<br> | |||
B. [[File:2 B cell lym liver 1 680x512px.tif|Apparent inflamed fibrous band between two relatively hepatocyte regions (Row 1 Right 40X).]] | |||
<br> | |||
C. [[File:3 B cell lym liver 1 680x512px.tif|Apparent piecemeal necrosis with bile ductular proliferation (Row 2 Left 200X).]] | |||
<br> | |||
D. [[File:4 B cell lym liver 1 680x512px.tif|Apparent portal inflammation with unaffected interlobular bile duct (Row 2 Right 200X).]] | |||
<br> | |||
E. [[File:5 B cell lym liver 1 680x512px.tif|Apparent lobular infiltrate with small masse.]] | |||
<br> | |||
F. [[File:6 B cell lym liver 1 680x512px.tif|Proof is at high power. All cells are similar to macrophages but are too closely crowded to be macrophages. The monomorphism (one type of cell) should inspire immunohistochemical stains, which showed the patient had a B cell lymphoma.]] | |||
<br> | |||
B cell lymphoma mimicking hepatitis with fibrosis. A. Apparent inflamed fibrous tract with lobular inflammatory collections in adjacent liver. B. Apparent inflamed fibrous band between two relatively hepatocyte regions. C. Apparent piecemeal necrosis with bile ductular proliferation. D. Apparent portal inflammation with unaffected interlobular bile duct. E. Apparent lobular inflammation with collections a bit too large for usual lobular inflammation. F. Proof is at high power. All cells are similar to macrophages but are too closely crowded to be macrophages. The monomorphism (one type of cell) should inspire immunohistochemical stains, which showed the patient had a B cell lymphoma. | |||
[[File:5 02965636298621 sl 1.png|Malignant B cell lymphoma, NOS, in a 63 year old man’s liver]] | |||
[[File:5 02965636298621 sl 2.png|Malignant B cell lymphoma, NOS, in a 63 year old man’s liver]] | |||
[[File:5 02965636298621 sl 3.png|Malignant B cell lymphoma, NOS, in a 63 year old man’s liver]] | |||
[[File:5 02965636298621 sl 4.png|Malignant B cell lymphoma, NOS, in a 63 year old man’s liver]] | |||
[[File:5 02965636298621 sl 31.png|Malignant B cell lymphoma, NOS, in a 63 year old man’s liver]] | |||
[[File:5 02965636298621 sl 6.png|Malignant B cell lymphoma, NOS, in a 63 year old man’s liver]] | |||
<br> | <br> | ||
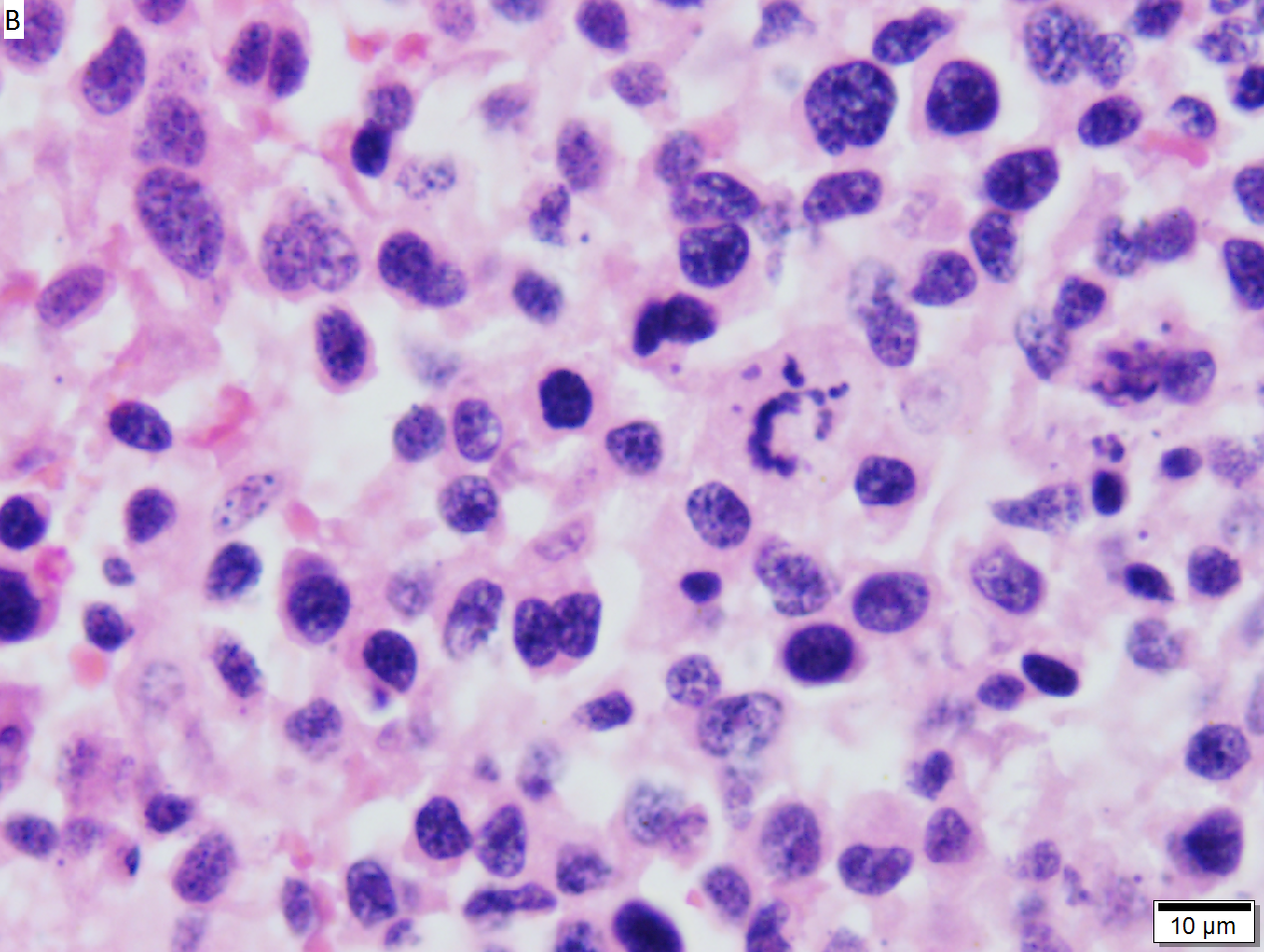
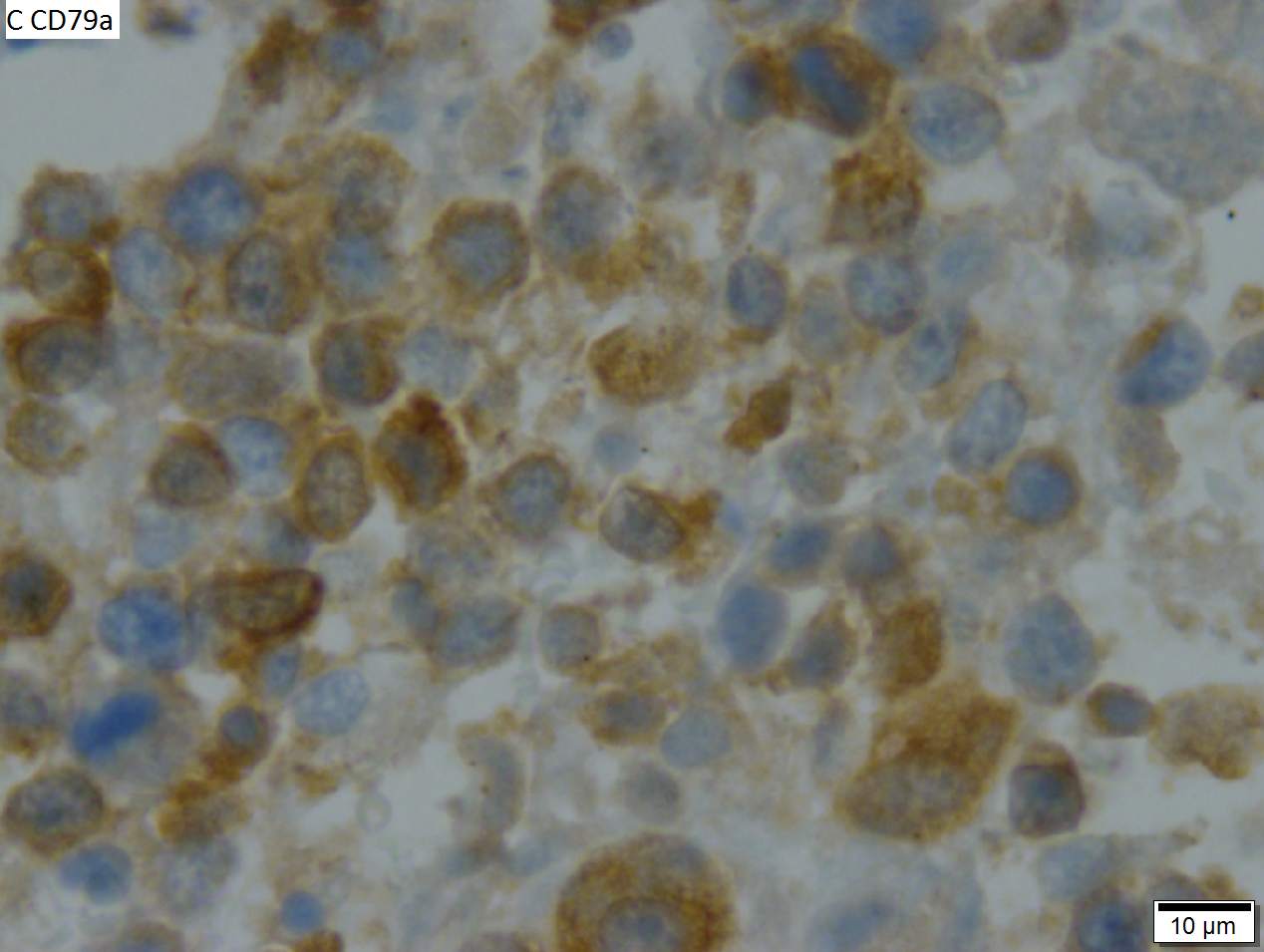
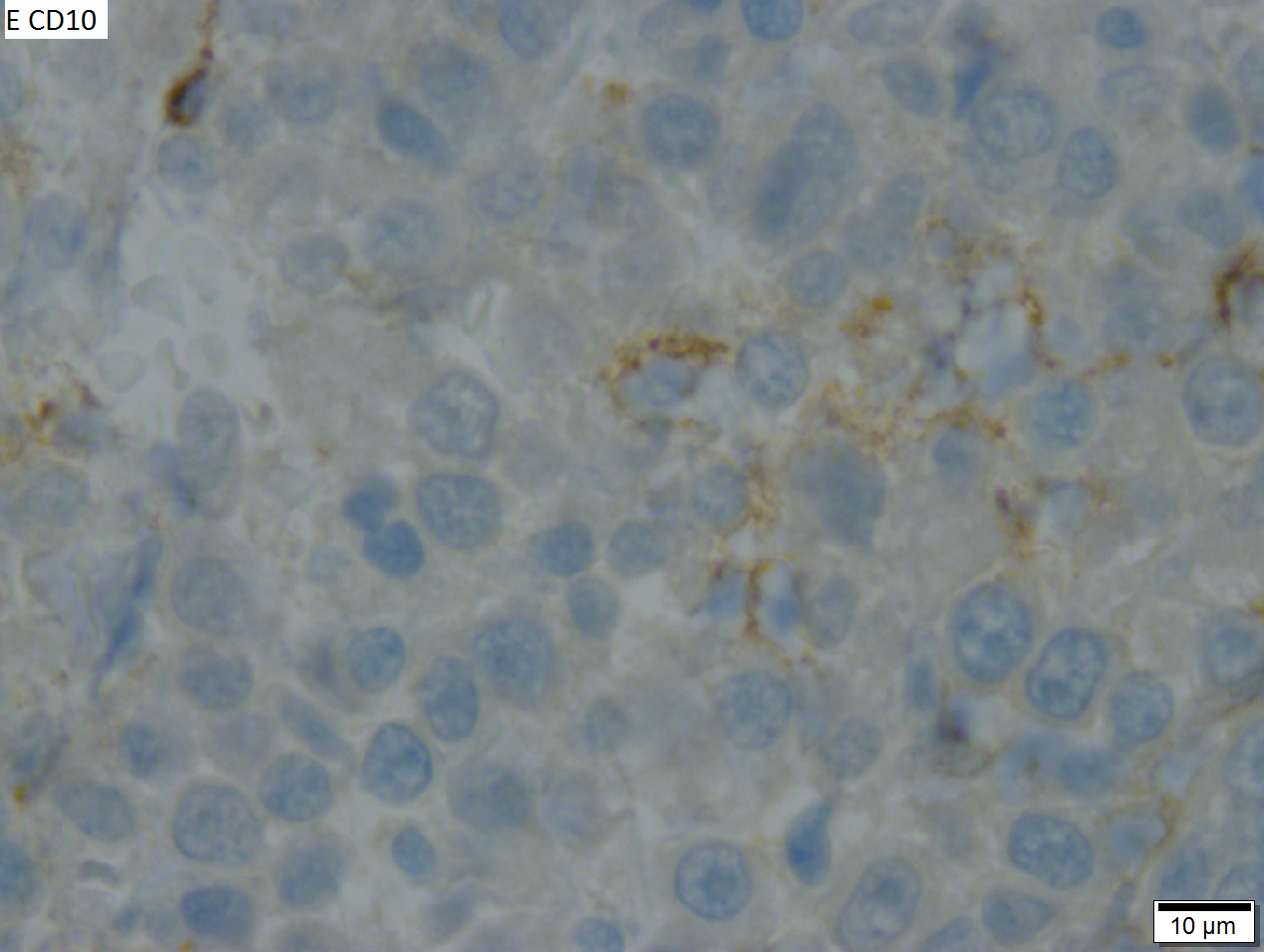
Malignant B cell lymphoma, NOS, in a 63 year old man’s liver. No other specimens were available for further classification. A. Tumor expands a triad and occupies parenchymal regions. B. Bounding a bile duct, modestly sized round to reniform lymphoid cells, many without nucleoli, accompany small round lymphocytes. Some of the larger cells have clefts (arrows). C. CD3 stain shows many of the lymphoid cells are intercalated reactive T cells. D. Ki67 shows less than half the tumor cells, mostly the larger ones, are in proliferative phase, arguing against the notion of a high grade B cell lymphoma. E. CD79A establishes B cell phenotype (CD20 was also positive). F. That the tumor cells are BCL2 positive evinces B cell neoplasia. The cells were CD10, BCL6, and cyclin D1 negative, militating against mantle cell lymphoma and CLL, with no follicular origin identified. | |||
[[File:4 89735893919405 sl 1.png| High grade B cell lymphoma involving liver]] | |||
[[File:4 89735893919405 sl 2.png| High grade B cell lymphoma involving liver]] | |||
[[File:4 89735893919405 sl 3.png| High grade B cell lymphoma involving liver]] | |||
[[File:4 89735893919405 sl 4.png| High grade B cell lymphoma involving liver]] | |||
[[File:4 89735893919405 sl 5.png| High grade B cell lymphoma involving liver]]<br> | |||
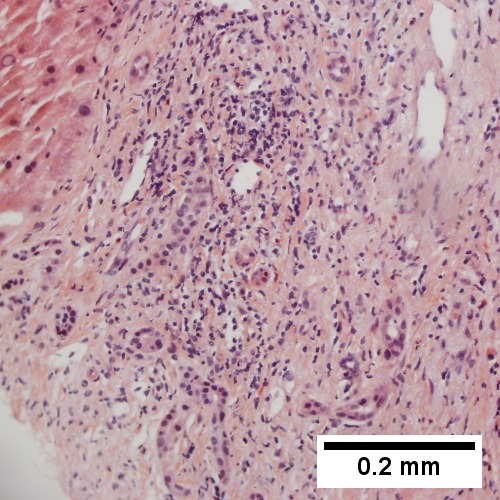
High grade B cell lymphoma involving liver in a 77 year old woman. A. A band of cancer abuts fibrotic liver with steatosis. B. Cancer cells show primitive, round to ovoid, variably sized, dark nucleoli and an aberrant mitoses. Cytoplasm is scant. C. Cancer cells are CD79a positive. D. Most cancer cell nuclei were positive for Ki-67, overall about 80%. E. A minority of cancer cells are CD10 positive. | |||
=See also= | |||
*[[Pancreas]]. | *[[Pancreas]]. | ||
*[[Gastrointestinal pathology]]. | *[[Gastrointestinal pathology]]. | ||
*[[Liver pathology]]. | *[[Liver pathology]]. | ||
=References= | |||
{{reflist|2}} | {{reflist|2}} | ||
[[Category:Gastrointestinal pathology]] | [[Category:Gastrointestinal pathology]] | ||
[[Category:Liver pathology]] | [[Category:Liver pathology]] | ||
Latest revision as of 23:15, 31 January 2017
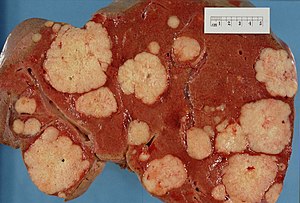
This article examines liver neoplasms and pre-malignant lesions of the liver. In North America, most malignant liver lesions are metastases.
This article focuses on primary malignancies of the liver, neoplastic liver lesions, and biliary malignancies. It only briefly discusses metastatic lesions. An introduction to liver pathology is in the liver article. Medical liver disease is dealt with in the medical liver disease article.
Overview
Dysplasic lesions of the liver
Types:[1]
- "Large cell dysplasia" (AKA large cell change) - not considered a precursor for HCC, not considered a dysplasia.[2]
- Small liver cell dysplasia (AKA small cell dysplasia).
- Low grade dysplasia.
- High grade dysplasia.
Neoplastic lesions
Malignant lesions of the liver
- Hepatocellular carcinoma (HCC) - most common malignant liver primary in adults.
- Hepatoblastoma - malignant liver primary in children.
- Intrahepatic cholangiocarcinoma (ICC).[3]
- Combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma (CHC).
Lesions that arise in a non-cirrhotic liver
Hepatocellular:
Other:
Tabular comparison
Precursors
Features of HCC & its precursors - generated from DCHH[4] and STC:
| Features | SLCD | Low-grade dysplasia | High-grade dysplasia | HCC |
| Plate thickness | <3 cells | <=2 cells | <=3 cells, usu. >2 cells | >3 cells |
| Reticulin (stain) | intact chicken wire | intact chicken wire | intact chicken wire | damaged chicken wire |
| Nuclear changes | nuc. enlargement, hyperchromasia |
+/- atypia (???) | marked atypia | +/- incr. NCR, +/-irreg. nuc. contour |
| Cytoplasmic change | hyperchromasia, decr. as cell size preserved |
none (???) | +/- basophilia | variable (lighter vs. hyperchromasia) |
| Portal tracts | ? | loss of portal tracts | loss of portal tracts | loss of portal tracts |
| Management | follow ??? | follow | ablate | ablate/surgery |
Abbreviations:
- SLCD = small liver cell dysplasia.
Notes:
- Large cell dysplasia:
- Cell size ~ 2x normal, NC ratio ~ normal.
- SLCD:
- Cell size ~ 1/2x normal, NC ratio - increased.
Hepatic tumours
Benign:
| Entity | Gross | Microscopic | IHC/stains | Other | Images |
|---|---|---|---|---|---|
| Hepatic hemangioma | similar to normal liver parenchyma, red (hemorrhagic), well-circumscribed | spaces lined by benign endothelial cells | CD31+ (???) | - | gross (rsna.org) |
| Focal nodular hyperplasia | central scar, large vessels, usu. well-circumscribed | large arteries, unpaired arteries, bile duct proliferation | usu. diagnosed by imaging | gross (rsna.org) | |
| Hepatocellular adenoma | subcapsular, well-circumscribed | loss of portal tracts, nuclear glycogenation | reticulin - liver plate thickness <= 3 | background not cirrhotic, assoc. OCP | gross (mda-sy.com)[5] |
Malignant:
| Entity | Gross | Microscopic | IHC/stains | Other | Images |
|---|---|---|---|---|---|
| Liver metastasis | multiple, white lesions | variable, usu. tubular (glandular) with pseudostratified hyperchromatic nuclei | CK7-, CK20+ (colorectal), HepPar-1-, CK19- | colorectal carcinoma most common | |
| Hepatocellular carcinoma | poorly circumscribed, +/-necrosis, +/-hemorrhage | loss of portal tracts, unpaired arteries, +/-nuclear atypia | reticulin - liver plate thickness > 3 | background often cirrhotic | |
| Cholangiocarcinoma | cauliflower-like outline, white, classically solitary, no cirrhosis | tubular architecture and mild nuclear atypia (adenocarcinoma), desmoplastic stroma | CK7+, CK19+ | background usu. not cirrhotic |
Dysplasia of the liver
Small liver cell dysplasia
- Abbreviated SLCD.
- AKA small cell dysplasia.
General
- Considered a precursor to HCC.
- Frequently found in livers with HCC - when compared to livers without HCC.[6]
Microscopic
Features:[7]
- Cells similar in size to normal hepatocytes.
- Name derived from the fact that there is also an entity that was called large cell dysplasia (AKA large liver cell dysplasia,[6] and large cell change).
- Increased NC ratio - "more blue".
- Mild nuclear and cytoplasmic hyperchromatism.
Notes:
- Normal hepatic architecture (main differentiator from HCC).
- Remember "... blue is bad".
Micrograph:
Low-grade hepatocellular dysplasia
- Generally referred to as low-grade dysplasia as the context is usually clear.
Microscopic
- Uniform cells - "noticeably different from normal".[8]
- Changes in nuclear size, irregular nuclear contour and/or changes in cytoplasm staining.
- Loss of portal tracts.
- Irregular margin.
Notes:
- DCHH describes LGD as: "normal hepatocytes in plates [of normal thickness]".[4]
DDx:
- Nodular regenerative hyperplasia - lacks: compressed rim of cells, central portal tract.[4]
High-grade hepatocellular dysplasia
- Generally referred to as high-grade dysplasia as the context is usually clear.
General
- "Bader" version of low-grade dyplasia.
Microscopic
Features - in addition to those of low grade dysplasia:[4]
- Liver plate >2 cells thick.
- Significant nuclear atypia.
- Basophilic cytoplasm.
DDx:
Image:
Benign hepatic neoplasms
Bile duct hamartoma
Bile duct hamartomas. A. Trichrome shows fibrous spaces with dilated ducts. B. Bizarre, ramifying tubules with dilatations. C. Bland epithelial linings. D. Surrounding tract with tortuous bile ducts & inflammation, likely secondary to hamartomas.
Bile duct adenoma
- Should not be confused with bile duct hamartoma.
Hepatic adenoma
- AKA hepatocellular adenoma, abbreviated HCA.
Hepatobiliary mucinous cystadenoma
- AKA biliary cystadenoma.
General
- Benign neoplasm.
- May transform into a malignancy.[9]
Microscopic
Features:
- Cystic spaces lined by a mucinous epithelium (simple columnar epithelium with a clear cytoplasm).
- Surrounding dense ovarian like stroma.
DDX: Biliary Intraductal Papillary Neoplasm
- no surrounding ovarian stroma
- Intraductal - connects with the biliary tree lumen.
Note:
- Similar to pancreatic mucinous cystadenoma.
Cavernous hemangioma
Cavernous hemangioma. A. Fibrous foci with increased spaces, hepatocyte focus with nonspecific fibrotic bridge. B. Cavernous hemangioma with flat, non-atypical endothelium. C. Tortuous bile ductules, not to be considered generalized in presence of mass. D. Tortuous bile ducts, not to be considered generalized in presence of mass.
Malignant hepatic neoplasms
In North America, the most common malignant liver tumour is metastases.
Hepatoblastoma
General
- Most common liver cancer in children.[10][11]
- Rare in adolescents and adults.
- Age of diagnosis usu. ~1 year old; most less than 3 years old.
- Surgical biopsy; core needle biopsy not done as as lesion is vascular.
Associations:
Clinical:
- Usually present with hepatomegaly.
- High AFP.[13]
Microscopic
Features:
- Small round cell tumour.
- Fetal hepatocytes ~ 1:3 NC ratio, eosinophilic cytoplasm.
- +/-Mesenchymal component
- Immature fibrous tissue, osteoid or cartilage.
DDx:
- Small round cell tumours.
- Teratoma.
- Hepatocellular carcinoma - separated based on histomorphology alone.
Images
Subtypes
- Six histologic subtypes - that are subdivided into two groups:[14]
- Epithelial type:
- Fetal pattern.
- Embryonal and fetal pattern.
- Macrotrabecular pattern.
- May mimic hepatocellular carcinoma histologically.[15]
- Small cell undifferentiated pattern.
- Poor prognosis.
- Mixed epithelial and mesenchymal type:
- With teratoid features.
- Without teratoid features.
- Epithelial type:
IHC
- Alpha-fetoprotein +ve.
- Hepatocyte specific antigen +ve esp. in fetal component.[16]
- Beta-catenin +ve (cytoplasmic and nuclear).[16]
Hepatocellular carcinoma
- Abbreviated HCC.
Biliary Intraductal Papillary Neoplasm[17]
General
- Rare
- Highest incidence in Far Eastern countries
- Association with hepatolithiasis and clonorchiasis
- Between 50 and 70 years of age
- Slight male predominance
- Intermittent abdominal pain
- Acute cholangitis
- Jaundice
- Biliary counterpart of pancreatic intraductal papillary mucinous neoplasm
- Biliary counterpart of intracholecystic papillary neoplasm (gall bladder)
- Construct consumes some cases of biliary cystadenoma/cystadenocarcinoma, biliary papilloma/papillomatosis, intraductal growth type of cholangiocarcinoma and papillary carcinoma of the extrahepatic bile duct.
Radiology
- Bile duct dilatation
- Intraductal masses
Gross
- Singular, or occasionally multiple, polypoid masses extending into the lumen of a dilated bile duct
- Or multilocular well-defined cystic mass containing mucinous fluid
- Granular or papillary mucosa
- Communication with bile duct may be difficult to confirm
Microscopic
- Papillary or villous growth within the lumen of an intra or extrahepatic bile duct
- Papillary fronds with fine vascular cores
- Epithelium types
- Pancreatobiliary
- Intestinal - marked mucin secretion
- Gastric
- Oncocytic types
- Dysplasia
- High or low grade
- Marked variation in histologic grade between different regions of individual tumors
- Common association with invasive cholangiocarcinoma
- Tubular adenocarcinoma
- Mucinous (colloid) carcinoma (often in association with the intestinal type).
DDX
- Biliary Mucinous Cystic Neoplasm
- Epithelium is surrounded by a distinct ovarian-like stroma.
Photos
Intraductal papillary neoplasm of common bile duct with associated invasive carcinoma. A. The papillary tumor comprises mostly variably dilated acini. B. Tumor also shows areas of micropapillae. C. Some areas within the non-invasive tumor showed necrosis, with the black pyknotic nuclei amid red debris. D. Definite invasion was established low power by glands headed in perpendicular directions. E. Embedded in fibroblastic response are non-acinar walls and isolated epithelial groups. F. Also embedded in fibroblastic response are flat glands with nuclei showing loss of polarity (lack of respect for lateral intercellular borders shown by variable orientation to base of gland).
Notes -
- Reflect on the known marked variation in histologic grade between different regions of individual tumors when rendering an opinion on a small biopsy specimen.
- Consider the possibility of an invasive component and submit tissue generously.
See also: PubCan [1]
Cholangiocarcinoma
Hepatic angiosarcoma
- AKA angiosarcoma of the liver.
General
- Liver angiosarcomas are associated with vinyl chloride exposure.[19]
Microscopic
Features:
- Atypical endothelial cells - may be subtle.
Hepatic metastasis
Hematopoietic tumors
A 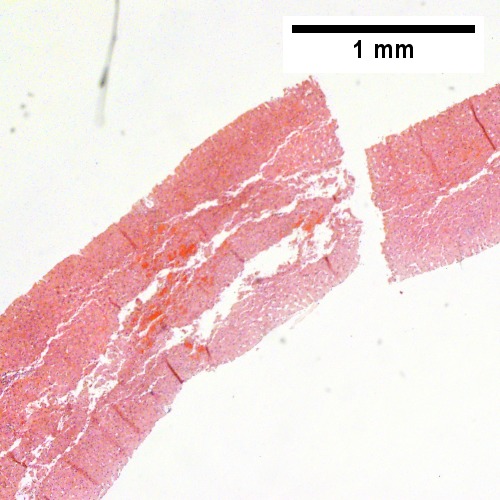
B 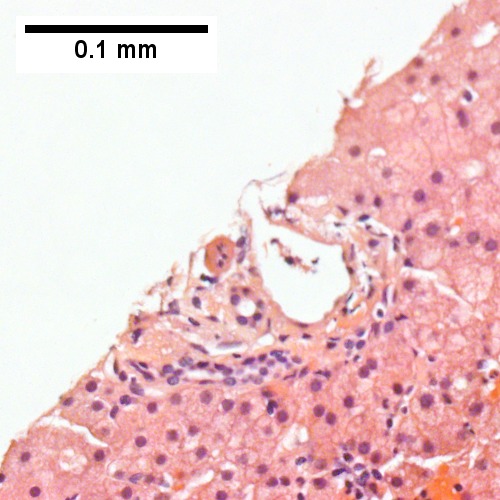
C 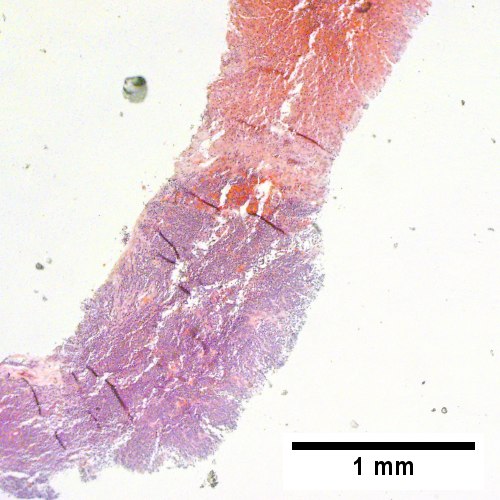
D 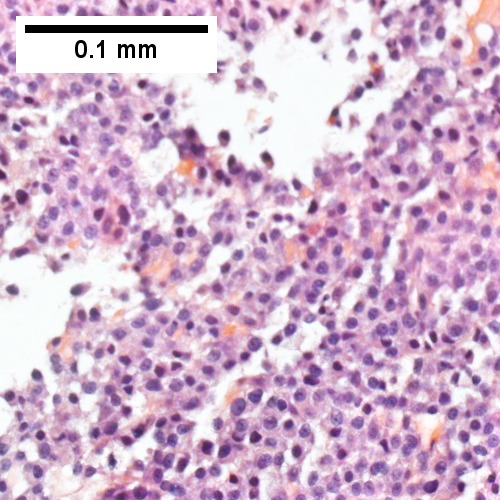
Plasmacytoma appearing as a tumor mass. A. One liver core was normal. B. A triad with a proliferated bile ductule, otherwise normal. C. The other core showed a mass of tumor mashed against normal liver. D. Tumor cells showed round to ovoid nuclei without pattern and with grey cytoplasm that proved to be CD138 positive.
A. 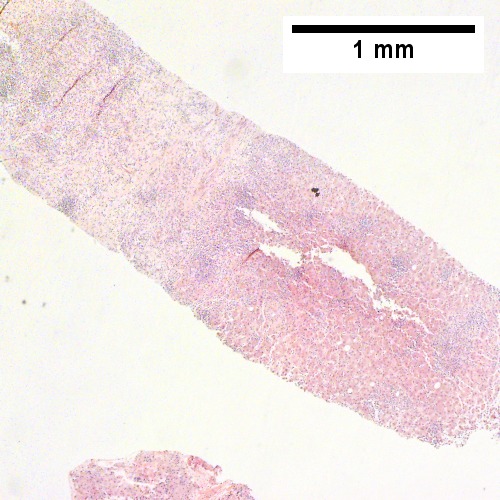
B. 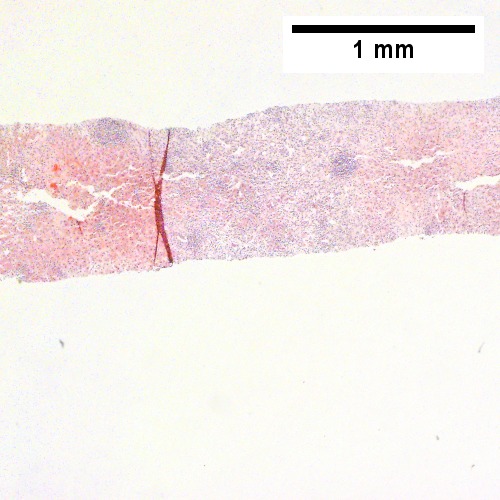
C. 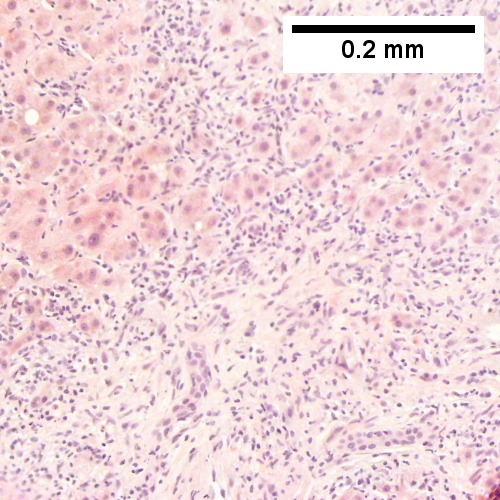
D. 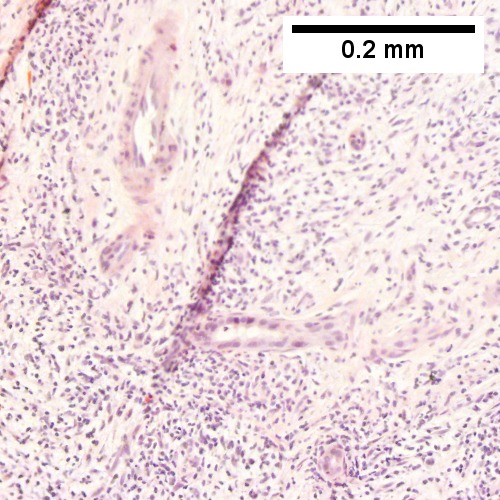
E. 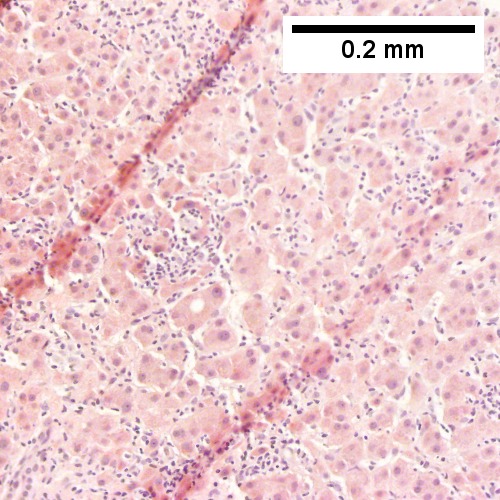
F. 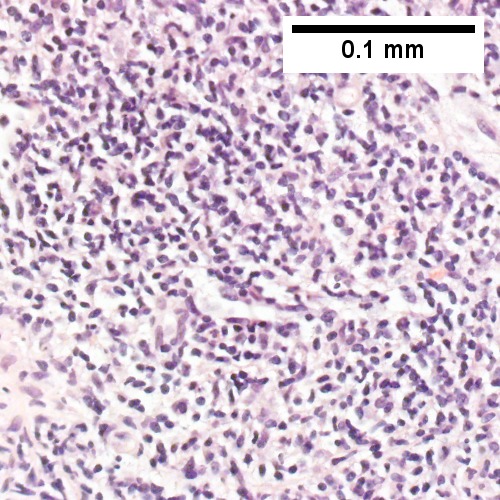
B cell lymphoma mimicking hepatitis with fibrosis. A. Apparent inflamed fibrous tract with lobular inflammatory collections in adjacent liver. B. Apparent inflamed fibrous band between two relatively hepatocyte regions. C. Apparent piecemeal necrosis with bile ductular proliferation. D. Apparent portal inflammation with unaffected interlobular bile duct. E. Apparent lobular inflammation with collections a bit too large for usual lobular inflammation. F. Proof is at high power. All cells are similar to macrophages but are too closely crowded to be macrophages. The monomorphism (one type of cell) should inspire immunohistochemical stains, which showed the patient had a B cell lymphoma.


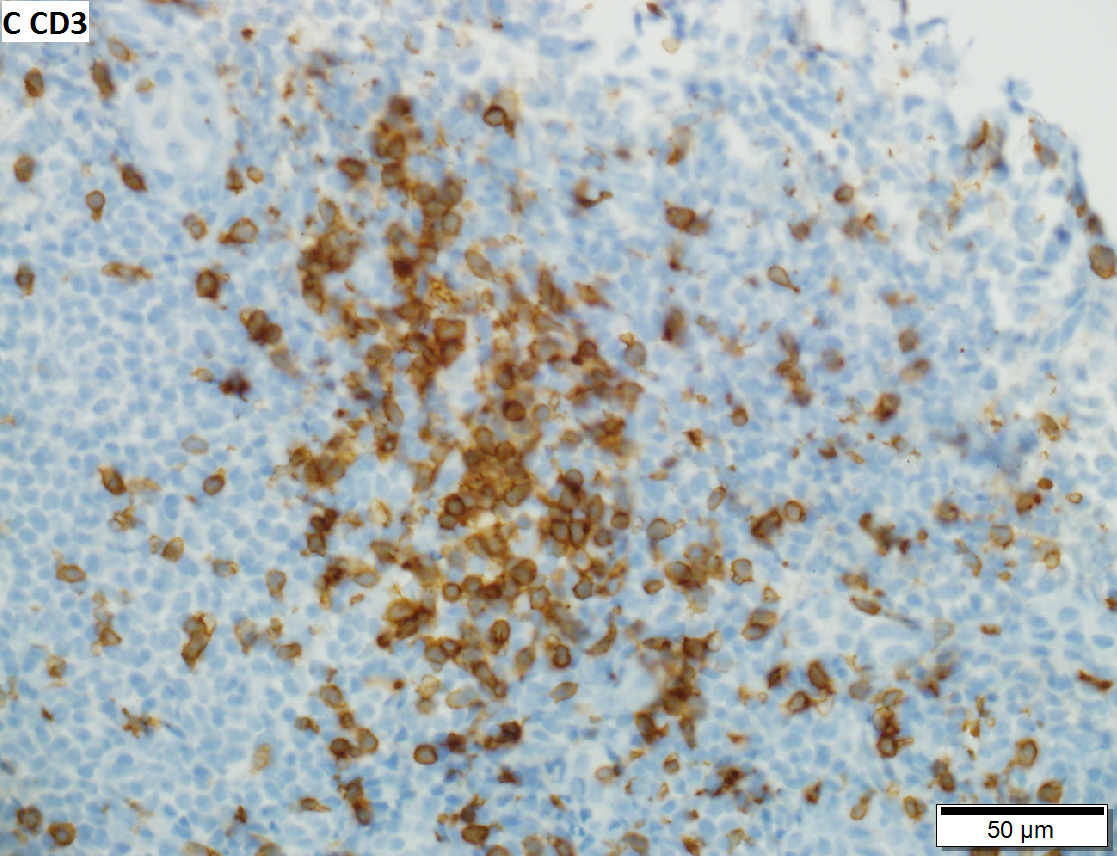


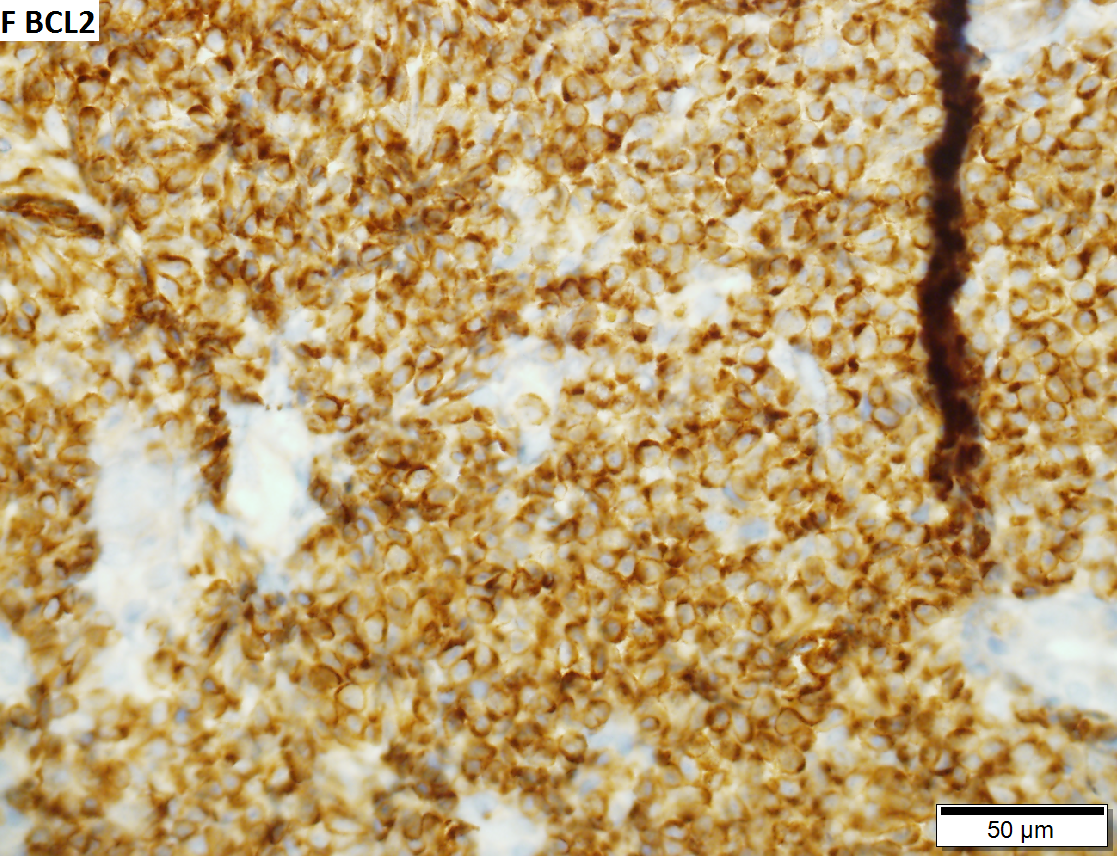
Malignant B cell lymphoma, NOS, in a 63 year old man’s liver. No other specimens were available for further classification. A. Tumor expands a triad and occupies parenchymal regions. B. Bounding a bile duct, modestly sized round to reniform lymphoid cells, many without nucleoli, accompany small round lymphocytes. Some of the larger cells have clefts (arrows). C. CD3 stain shows many of the lymphoid cells are intercalated reactive T cells. D. Ki67 shows less than half the tumor cells, mostly the larger ones, are in proliferative phase, arguing against the notion of a high grade B cell lymphoma. E. CD79A establishes B cell phenotype (CD20 was also positive). F. That the tumor cells are BCL2 positive evinces B cell neoplasia. The cells were CD10, BCL6, and cyclin D1 negative, militating against mantle cell lymphoma and CLL, with no follicular origin identified.


High grade B cell lymphoma involving liver in a 77 year old woman. A. A band of cancer abuts fibrotic liver with steatosis. B. Cancer cells show primitive, round to ovoid, variably sized, dark nucleoli and an aberrant mitoses. Cytoplasm is scant. C. Cancer cells are CD79a positive. D. Most cancer cell nuclei were positive for Ki-67, overall about 80%. E. A minority of cancer cells are CD10 positive.
See also
References
- ↑ STC. S.30-37, 19 Jan 2009.
- ↑ Park, YN.; Roncalli, M. (Nov 2006). "Large liver cell dysplasia: a controversial entity.". J Hepatol 45 (5): 734-43. doi:10.1016/j.jhep.2006.08.002. PMID 16982109.
- ↑ Shirakawa, H.; Kuronuma, T.; Nishimura, Y.; Hasebe, T.; Nakano, M.; Gotohda, N.; Takahashi, S.; Nakagohri, T. et al. (Mar 2009). "Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer.". Int J Oncol 34 (3): 649-56. PMID 19212669. http://www.spandidos-publications.com/serveFile/ijo_34_3_649_PDF.pdf?type=article&article_id=ijo_34_3_649&item=PDF.
- ↑ 4.0 4.1 4.2 4.3 Tadrous, Paul.J. Diagnostic Criteria Handbook in Histopathology: A Surgical Pathology Vade Mecum (1st ed.). Wiley. pp. 170-1. ISBN 978-0470519035.
- ↑ URL: http://www.mda-sy.com/vb/showthread.php?p=5083&langid=1. Accessed on: 16 February 2012.
- ↑ 6.0 6.1 Szczepański, W. (1997). "Liver cell dysplasia in liver cirrhosis and hepatocellular carcinoma.". Pol J Pathol 48 (3): 147-57. PMID 9401407.
- ↑ STC S.32, 19 Jan 2009.
- ↑ STC - 19 Jan 2009. (???)
- ↑ Yu, J.; Wang, Y.; Yu, X.; Liang, P.. "Hepatobiliary mucinous cystadenoma and cystadenocarcinoma: report of six cases and review of the literature.". Hepatogastroenterology 57 (99-100): 451-5. PMID 20698207.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 923. ISBN 0-7216-0187-1.
- ↑ URL: http://emedicine.medscape.com/article/986802-overview. Accessed on: 29 November 2009.
- ↑ DeBaun MR, Tucker MA (March 1998). "Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry". J. Pediatr. 132 (3 Pt 1): 398–400. PMID 9544889.
- ↑ URL: http://emedicine.medscape.com/article/986802-diagnosis. Accessed on: 11 February 2011.
- ↑ URL: http://emedicine.medscape.com/article/986802-diagnosis. Accessed on: 11 February 2011.
- ↑ URL: http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=cap_foundation%2FcaseOfMonth%2FMar10%2Fmar_2010_cotm_diagnosis.html&_state=maximized&_pageLabel=cntvwr#null. Accessed on: 11 February 2011.
- ↑ 16.0 16.1 Halász, J.; Holczbauer, A.; Páska, C.; Kovács, M.; Benyó, G.; Verebély, T.; Schaff, Z.; Kiss, A. (May 2006). "Claudin-1 and claudin-2 differentiate fetal and embryonal components in human hepatoblastoma.". Hum Pathol 37 (5): 555-61. doi:10.1016/j.humpath.2005.12.015. PMID 16647953.
- ↑ .
- ↑ URL: http://www.cancer.org/cancer/bileductcancer/detailedguide/bile-duct-cancer-what-is-bile-duct-cancer. Access on: 23 May 2013.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 212. ISBN 978-1416054542.