Difference between revisions of "Diffuse astrocytoma"
Jump to navigation
Jump to search
Jensflorian (talk | contribs) (+pic) |
Jensflorian (talk | contribs) (→Molecular: CDKN2AB) |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
* Usually shows progression to [[glioblastoma]] sooner or later. | * Usually shows progression to [[glioblastoma]] sooner or later. | ||
WHO 2016 categorization combines morphology and genetics into following groups:<ref>{{Cite journal | last1 = Louis | first1 = DN. | last2 = Perry | first2 = A. | last3 = Reifenberger | first3 = G. | last4 = von Deimling | first4 = A. | last5 = Figarella-Branger | first5 = D. | last6 = Cavenee | first6 = WK. | last7 = Ohgaki | first7 = H. | last8 = Wiestler | first8 = OD. | last9 = Kleihues | first9 = P. | title = The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. | journal = Acta Neuropathol | volume = 131 | issue = 6 | pages = 803-20 | month = Jun | year = 2016 | doi = 10.1007/s00401-016-1545-1 | PMID = 27157931 }}</ref> | |||
*Diffuse astrocytoma ICD-O: 9400/3 | *Diffuse astrocytoma, IDH-mutant ICD-O: 9400/3 - most frequent. | ||
** | **Gemistocytic astrocytoma, IDH-mutant ICD-O:9411/3 | ||
* | *Diffuse astrocytoma, IDH-wildtype ICD-O: 9400/3 | ||
* | *Diffuse astrocytoma,NOS ICD-O: 9400/3 - genetic data missing. | ||
Note: This subtyping is no longer in use | |||
''Note:'' Older terminologies included Fibrillary astrocytoma (ICD-O: 9420/3) and Protoplasmatic astrocytoma (ICD-O:9410/3)<ref name=WHOCNS>{{Ref WHOCNS|25}}</ref> This subtyping is no longer in use. These tumors are now classified according their IDH mutation status. | |||
==Radiology/Clinic== | |||
*Mass effect. | |||
*Seizures. | |||
*Neurologic decifit. | |||
*Usually not contrast-enhanching, T2 bright. | |||
==Macroscopy== | |||
*No clear demarcation from white matter | |||
*May contain larger cysts | |||
*No necrosis | |||
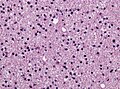
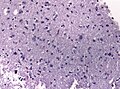
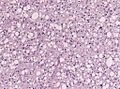
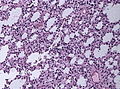
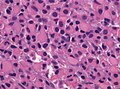
==Histology== | ==Histology== | ||
Features: <ref name=AFIP2007>{{Ref AFIP2007|34}}</ref> | |||
*Cell density higher than normal brain. | *Cell density higher than normal brain. | ||
*Mild to moderate nuclear pleomorphism. | *Mild to moderate nuclear pleomorphism. | ||
**Monotony of atypical nuclei | **Monotony of atypical nuclei and irregular distribution indicates neoplasm. | ||
**"naked nuclei" without recognizeable processes. | |||
**No prominent nucleolus. | |||
*Cytoplasm highly variable (even within the same tumour). | *Cytoplasm highly variable (even within the same tumour). | ||
**In normal CNS the cytoplasm blends within the neuropil. | **In normal CNS the cytoplasm blends within the neuropil. | ||
*Mitoses absent or very rare. | *Mitoses absent or very rare. | ||
*Microcystic | *Microcystic spaces of the background (none to extensive). | ||
*No necrosis, no vascular proliferations. | *No necrosis, no vascular proliferations. | ||
**Except radiation necrosis. | |||
*Lymphocytic cuffing (mostly in gemistocytic type) | |||
*Abent to few rosenthal fibers. | |||
<gallery> | |||
File:Diffuse_astrocytoma_HE_stain.jpg | Diffuse astrocytoma, [[H&E]] (WC/jensflorian) | |||
File:Image NP T2a 0002.JPG | Diffuse astrocytoma, [[H&E]] (WC/jensflorian) | |||
File:Astrocytoma whoII HE.jpg | Astrocytoma, fibrillary type (WC/jensflorian) | |||
File:Neuropathology case II 02.jpg | Astrocytoma, protoplasmatic type (WC/jensflorian) | |||
File:Gemistocytic astrocytoma.jpg | Gemistocytic astrocytoma (WC/jensflorian) | |||
</gallery> | |||
==IHC== | ==IHC== | ||
| Line 27: | Line 54: | ||
*Vimentin+ve (often perinuclear). | *Vimentin+ve (often perinuclear). | ||
*S-100+ve. | *S-100+ve. | ||
*p53: Nuclear staining in 30% of the tumours (usually few cells). | |||
*MIB-1: 0-5% (mean: 2%). | *MIB-1: 0-5% (mean: 2%). | ||
*[[IDH-1]] (R132H)+ve in 60-70%. | *[[IDH-1]] (R132H)+ve in 60-70%. | ||
*[[ATRX]] loss in 70%. | **'Note:'' This antibody does not detect other rare IDH1/2 mutations. | ||
*[[ATRX]] nuclear loss in 70%. | |||
<gallery> | |||
File:GFAP astrocytoma.jpg| GFAP in astrocytoma (WC/jensflorian) | |||
File:Neuropathology case II 04.jpg | ATRX loss in astrocytoma (WC/jensflorian) | |||
</gallery> | |||
==Molecular== | ==Molecular== | ||
*Absence of LOH 1p/19q. | *IDH1 R132- or IDH2 R172-hotsopt mutations classify the tumors as Diffuse astrocytoma, IDH-mutant. | ||
*Absence of LOH 1p/19q (otherwise classify tumor as oligodendroglioma). | |||
*Tp53 mutations in approx. 60% (80-90% in gemistocytic, 50% in fibrillary types). | *Tp53 mutations in approx. 60% (80-90% in gemistocytic, 50% in fibrillary types). | ||
*MGMT promotor methylated in approx. 50%. | *MGMT promotor methylated in approx. 50%. | ||
*CDKN2A/B homozygous deletion in IDH mutant diffuse astrocytoma has unfavourable prognosis.<ref>{{Cite journal | last1 = Shirahata | first1 = M. | last2 = Ono | first2 = T. | last3 = Stichel | first3 = D. | last4 = Schrimpf | first4 = D. | last5 = Reuss | first5 = DE. | last6 = Sahm | first6 = F. | last7 = Koelsche | first7 = C. | last8 = Wefers | first8 = A. | last9 = Reinhardt | first9 = A. | title = Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. | journal = Acta Neuropathol | volume = 136 | issue = 1 | pages = 153-166 | month = Jul | year = 2018 | doi = 10.1007/s00401-018-1849-4 | PMID = 29687258 }}</ref><ref>{{Cite journal | last1 = Aoki | first1 = K. | last2 = Nakamura | first2 = H. | last3 = Suzuki | first3 = H. | last4 = Matsuo | first4 = K. | last5 = Kataoka | first5 = K. | last6 = Shimamura | first6 = T. | last7 = Motomura | first7 = K. | last8 = Ohka | first8 = F. | last9 = Shiina | first9 = S. | title = Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. | journal = Neuro Oncol | volume = 20 | issue = 1 | pages = 66-77 | month = 01 | year = 2018 | doi = 10.1093/neuonc/nox132 | PMID = 29016839 }}</ref> | |||
Note: | |||
*The existence of diffuse astrocytoma, IDH wildtype is challenged.<ref>{{Cite journal | last1 = Reuss | first1 = DE. | last2 = Kratz | first2 = A. | last3 = Sahm | first3 = F. | last4 = Capper | first4 = D. | last5 = Schrimpf | first5 = D. | last6 = Koelsche | first6 = C. | last7 = Hovestadt | first7 = V. | last8 = Bewerunge-Hudler | first8 = M. | last9 = Jones | first9 = DT. | title = Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. | journal = Acta Neuropathol | volume = 130 | issue = 3 | pages = 407-17 | month = Sep | year = 2015 | doi = 10.1007/s00401-015-1454-8 | PMID = 26087904 }}</ref> | |||
**Most adult cases show genetic alterations compatible with glioblastoma.<ref>{{Cite journal | last1 = Hasselblatt | first1 = M. | last2 = Jaber | first2 = M. | last3 = Reuss | first3 = D. | last4 = Grauer | first4 = O. | last5 = Bibo | first5 = A. | last6 = Terwey | first6 = S. | last7 = Schick | first7 = U. | last8 = Ebel | first8 = H. | last9 = Niederstadt | first9 = T. | title = Diffuse Astrocytoma, IDH-Wildtype: A Dissolving Diagnosis. | journal = J Neuropathol Exp Neurol | volume = | issue = | pages = | month = Feb | year = 2018 | doi = 10.1093/jnen/nly012 | PMID = 29444314 }}</ref> | |||
**Molecular upgrade according to cIMPACT-NOW Update 3 consensus (one of these is sufficient):<ref>{{Cite journal | last1 = Brat | first1 = DJ. | last2 = Aldape | first2 = K. | last3 = Colman | first3 = H. | last4 = Holland | first4 = EC. | last5 = Louis | first5 = DN. | last6 = Jenkins | first6 = RB. | last7 = Kleinschmidt-DeMasters | first7 = BK. | last8 = Perry | first8 = A. | last9 = Reifenberger | first9 = G. | title = cIMPACT-NOW update 3: recommended diagnostic criteria for "Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV". | journal = Acta Neuropathol | volume = 136 | issue = 5 | pages = 805-810 | month = Nov | year = 2018 | doi = 10.1007/s00401-018-1913-0 | PMID = 30259105 }}</ref> | |||
***EGFR amplification | |||
***Combined whole chromosome 7 gain and whole chromosome 10 loss (+ 7/− 10) | |||
***TERT promoter mutation | |||
**Suggested Sign-out: <pre> Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV</pre> | |||
**WHO grade II diffuse gliomas IDH-wt/H3-wt in children and adolescents have an indolent clinical behavior and rare anaplastic progression. | |||
***Most tumors show a BRAFV600E mutation, an FGFR alteration, or a MYB or MYBL1 rearrangement.<ref>{{Cite journal | last1 = Ellison | first1 = DW. | last2 = Hawkins | first2 = C. | last3 = Jones | first3 = DTW. | last4 = Onar-Thomas | first4 = A. | last5 = Pfister | first5 = SM. | last6 = Reifenberger | first6 = G. | last7 = Louis | first7 = DN. | title = cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF | journal = Acta Neuropathol | volume = 137 | issue = 4 | pages = 683-687 | month = Apr | year = 2019 | doi = 10.1007/s00401-019-01987-0 | PMID = 30848347 }}</ref> | |||
***Glial morphology can be astrocytic or oligodendrocytic. | |||
**Suggested sign-out: | |||
<pre> | |||
Diffuse glioma, MYB-altered. | |||
Diffuse glioma, MYBL1-altered. | |||
Diffuse glioma, FGFR1 TKD-duplicated. | |||
Diffuse glioma, FGFR1-mutant. | |||
Diffuse glioma, BRAF V600E-mutant. | |||
Diffuse glioma, other MAPK pathway alteration. | |||
</pre> | |||
==DDx== | ==DDx== | ||
*Reactive astrocytosis. | *Reactive astrocytosis. | ||
*Demyelinisation. | *Demyelinisation. | ||
*[[Anaplastic astrocytoma]] | *[[Anaplastic astrocytoma]] - increased mitotic activity. | ||
*[[Oligoastrocytoma]] | *[[Oligoastrocytoma]], NOS - esp. when genetic data on IDH and LOH 1p/19q are lacking. | ||
*[[Oligodendroglioma]] - esp. protoplasmatic forms. | *[[Oligodendroglioma]] - esp. protoplasmatic forms. LOH 1p/19q testing required. | ||
*[[SEGA]] - esp. gemistocytic forms. | *[[SEGA]] - esp. gemistocytic forms. | ||
=See also= | =See also= | ||
Latest revision as of 08:12, 27 May 2019
Diffuse astrocytoma (AKA: diffuse, low-grade astrocytoma) is a infiltrating astrocytoma occurring in the CNS white matter.
- Most common grade II WHO glioma in adults (peaks between 30-40 years).
- 10-15% of all astrocytomas.
- Usually shows progression to glioblastoma sooner or later.
WHO 2016 categorization combines morphology and genetics into following groups:[1]
- Diffuse astrocytoma, IDH-mutant ICD-O: 9400/3 - most frequent.
- Gemistocytic astrocytoma, IDH-mutant ICD-O:9411/3
- Diffuse astrocytoma, IDH-wildtype ICD-O: 9400/3
- Diffuse astrocytoma,NOS ICD-O: 9400/3 - genetic data missing.
Note: Older terminologies included Fibrillary astrocytoma (ICD-O: 9420/3) and Protoplasmatic astrocytoma (ICD-O:9410/3)[2] This subtyping is no longer in use. These tumors are now classified according their IDH mutation status.
Radiology/Clinic
- Mass effect.
- Seizures.
- Neurologic decifit.
- Usually not contrast-enhanching, T2 bright.
Macroscopy
- No clear demarcation from white matter
- May contain larger cysts
- No necrosis
Histology
Features: [3]
- Cell density higher than normal brain.
- Mild to moderate nuclear pleomorphism.
- Monotony of atypical nuclei and irregular distribution indicates neoplasm.
- "naked nuclei" without recognizeable processes.
- No prominent nucleolus.
- Cytoplasm highly variable (even within the same tumour).
- In normal CNS the cytoplasm blends within the neuropil.
- Mitoses absent or very rare.
- Microcystic spaces of the background (none to extensive).
- No necrosis, no vascular proliferations.
- Except radiation necrosis.
- Lymphocytic cuffing (mostly in gemistocytic type)
- Abent to few rosenthal fibers.
IHC
- GFAP+ve.
- MAP2+ve (especially in cell processes).
- Vimentin+ve (often perinuclear).
- S-100+ve.
- p53: Nuclear staining in 30% of the tumours (usually few cells).
- MIB-1: 0-5% (mean: 2%).
- IDH-1 (R132H)+ve in 60-70%.
- 'Note: This antibody does not detect other rare IDH1/2 mutations.
- ATRX nuclear loss in 70%.
Molecular
- IDH1 R132- or IDH2 R172-hotsopt mutations classify the tumors as Diffuse astrocytoma, IDH-mutant.
- Absence of LOH 1p/19q (otherwise classify tumor as oligodendroglioma).
- Tp53 mutations in approx. 60% (80-90% in gemistocytic, 50% in fibrillary types).
- MGMT promotor methylated in approx. 50%.
- CDKN2A/B homozygous deletion in IDH mutant diffuse astrocytoma has unfavourable prognosis.[4][5]
Note:
- The existence of diffuse astrocytoma, IDH wildtype is challenged.[6]
- Most adult cases show genetic alterations compatible with glioblastoma.[7]
- Molecular upgrade according to cIMPACT-NOW Update 3 consensus (one of these is sufficient):[8]
- EGFR amplification
- Combined whole chromosome 7 gain and whole chromosome 10 loss (+ 7/− 10)
- TERT promoter mutation
- Suggested Sign-out:
Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV
- WHO grade II diffuse gliomas IDH-wt/H3-wt in children and adolescents have an indolent clinical behavior and rare anaplastic progression.
- Most tumors show a BRAFV600E mutation, an FGFR alteration, or a MYB or MYBL1 rearrangement.[9]
- Glial morphology can be astrocytic or oligodendrocytic.
- Suggested sign-out:
Diffuse glioma, MYB-altered.
Diffuse glioma, MYBL1-altered.
Diffuse glioma, FGFR1 TKD-duplicated.
Diffuse glioma, FGFR1-mutant.
Diffuse glioma, BRAF V600E-mutant.
Diffuse glioma, other MAPK pathway alteration.
DDx
- Reactive astrocytosis.
- Demyelinisation.
- Anaplastic astrocytoma - increased mitotic activity.
- Oligoastrocytoma, NOS - esp. when genetic data on IDH and LOH 1p/19q are lacking.
- Oligodendroglioma - esp. protoplasmatic forms. LOH 1p/19q testing required.
- SEGA - esp. gemistocytic forms.
See also
- ↑ Louis, DN.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, WK.; Ohgaki, H.; Wiestler, OD. et al. (Jun 2016). "The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary.". Acta Neuropathol 131 (6): 803-20. doi:10.1007/s00401-016-1545-1. PMID 27157931.
- ↑ The International Agency for Research on Cancer (Editors: Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.) (2007). Pathology and Genetics of Tumours of Tumors of the Central Nervous System (IARC WHO Classification of Tumours) (4th ed.). Lyon: World Health Organization. pp. 25. doi:10.1007/s00401-007-0243-4. ISBN 978-9283224303.
- ↑ Burger, P.C.; Scheithauer, B.W. (2007). Tumors of the Central Nervous System (Afip Atlas of Tumor Pathology) (4th ed.). Washington: American Registry of Pathology. pp. 34. ISBN 1933477016.
- ↑ Shirahata, M.; Ono, T.; Stichel, D.; Schrimpf, D.; Reuss, DE.; Sahm, F.; Koelsche, C.; Wefers, A. et al. (Jul 2018). "Novel, improved grading system(s) for IDH-mutant astrocytic gliomas.". Acta Neuropathol 136 (1): 153-166. doi:10.1007/s00401-018-1849-4. PMID 29687258.
- ↑ Aoki, K.; Nakamura, H.; Suzuki, H.; Matsuo, K.; Kataoka, K.; Shimamura, T.; Motomura, K.; Ohka, F. et al. (01 2018). "Prognostic relevance of genetic alterations in diffuse lower-grade gliomas.". Neuro Oncol 20 (1): 66-77. doi:10.1093/neuonc/nox132. PMID 29016839.
- ↑ Reuss, DE.; Kratz, A.; Sahm, F.; Capper, D.; Schrimpf, D.; Koelsche, C.; Hovestadt, V.; Bewerunge-Hudler, M. et al. (Sep 2015). "Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities.". Acta Neuropathol 130 (3): 407-17. doi:10.1007/s00401-015-1454-8. PMID 26087904.
- ↑ Hasselblatt, M.; Jaber, M.; Reuss, D.; Grauer, O.; Bibo, A.; Terwey, S.; Schick, U.; Ebel, H. et al. (Feb 2018). "Diffuse Astrocytoma, IDH-Wildtype: A Dissolving Diagnosis.". J Neuropathol Exp Neurol. doi:10.1093/jnen/nly012. PMID 29444314.
- ↑ Brat, DJ.; Aldape, K.; Colman, H.; Holland, EC.; Louis, DN.; Jenkins, RB.; Kleinschmidt-DeMasters, BK.; Perry, A. et al. (Nov 2018). "cIMPACT-NOW update 3: recommended diagnostic criteria for "Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV".". Acta Neuropathol 136 (5): 805-810. doi:10.1007/s00401-018-1913-0. PMID 30259105.
- ↑ Ellison, DW.; Hawkins, C.; Jones, DTW.; Onar-Thomas, A.; Pfister, SM.; Reifenberger, G.; Louis, DN. (Apr 2019). "cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF". Acta Neuropathol 137 (4): 683-687. doi:10.1007/s00401-019-01987-0. PMID 30848347.