Oligodendroglioma
Revision as of 07:38, 15 May 2019 by Jensflorian (talk | contribs) (→Histologic grading: IDH1 and 2)
Oligodendroglioma is CNS tumour that is typically in the cerebral hemispheres.
| Oligodendroglioma | |
|---|---|
| Diagnosis in short | |
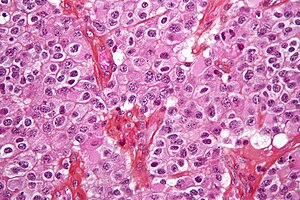 Oligodendroglioma. H&E stain. | |
|
| |
| LM | highly cellular lesion composed of cells resembling fried eggs (oligodendrocytes) with a round nucleus (important), distinct cell borders, +/-clear cytoplasm - useful feature (if present), acutely branched capillary sized vessels ("chicken-wire" like appearance), calcifications |
| LM DDx | neurocytoma, clear cell variant of ependymoma, seminoma / dysgerminoma / germinoma |
| Molecular | +/-loss of 1p and 19q (common) |
| Site | neuropathology tumours - cerebral hemispheres, posterior fossa (rare), spinal cord (very rare) |
|
| |
| Radiology | intra-axial mass, +/-calcifications (best seen on CT), nonenhancing or enhancing |
| Prognosis | moderate - dependent on grade |
General
- Do not arise from oligodendrocytes, although tumor cells look very similiar.[1]
- Arise from glial precursor cells.
Prognosis by flavours (average survival):[2]
- WHO grade II: 10-15 years.
- WHO grade III: 3-5 years.
Oligodendrogliomas account for approx 5% of all gliomas.
- Numbers may be higher when stringent classifiation criteria are not applied.
- Peak incidence: 40-45 years.
- First description of the tumor in 1926.
The WHO 2016 classification recognizes four tumor subtypes:[3]
- Oligodendroglioma, IDH-mutant and 1p/19q-codeleted, WHO Grade II (ICD-O: 9450/3).
- Anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted, WHO Grade III (ICD-O: 9451/3).
- Oligodendroglioma, NOS, WHO Grade II (ICD-O: 9450/3).
- Anaplastic oligodendroglioma, NOS, WHO Grade III (ICD-O: 9451/3).
Gross/radiologic
Location:
- Cerebral hemispheres - most often (50-60%) frontal lobe, followed by parietal and temporal lobes.[4]
- Posterior fossa (rare).
- Intramedullary spinal cord (very rare).
Radiologic features:[4]
- Intra-axial mass.
- +/-Calcifications (best seen on CT).
- Nonenhancing or enhancing.
- Occasionally well-circumscribed.
Clinical symptoms:
- Seizures.
- Intracranial pressure.
- Focal neurologic decifits.
- Cognitive changes.
Microscopic
Features:
- Diffusely growing tumor.
- Highly cellular lesion composed of:
- Cells resembling fried eggs (oligodendrocytes) with:
- Round nucleus - key feature.
- Distinct cell borders.
- Moderate-to-marked nuclear atypia.
- Clear cytoplasm - useful feature (if present).
- Some oligodendrogliomas have eosinophilic cytoplasm with focal perinuclear clearing.
- Acutely branched capillary sized vessels - "chicken-wire" like appearance.
- Abundant, delicate appearing; may vaguely resemble a paraganglioma at low power.
- Cells resembling fried eggs (oligodendrocytes) with:
- Calcifications - important feature.[5]
- Perifocal edema uncommon.
- Few tumors may exhibit eosinophilic granular bodies.
- Some tumors may show a Spongioblastoma-like growth pattern.
Anaplastic (grade III) criteria:[6]
- "Significant" or "brisk" mitotic activity.
- That means for most neuropathologists >= 6 mitoses per 10 HPF.
- Microvacular proliferation.
- Necrosis.
Note:
- Tumour cells may be plasmacytoid, i.e. have a plasma cell-like appearance.[7]
- Also called minigemistocytes.
- The are usually strong GFAP+ve.
DDx:
- Neurocytoma also have perinuclear clearing and well-defined cellular borders.
- Pineocytomatous/neurocytic rosettes = (irregular) rosette with a large meshwork of fibers (neuropil) at the centre.
- Clear cell ependymoma.
- Dysembryoplastic neuroepithelial tumour.
- Oligodendroglial-like cells in Pilocytic astrocytoma.
- Clear cell meningioma.
- Demyelinisation.
Notes:
- Few neural tumours have round nuclei - DDx:
- Oligodendroglioma.
- Lymphoma.
- Clear cell variant of ependymoma.
- Germ cell tumour (germinoma/dysgerminoma/seminoma).
Images
Perinuclear MAP2 immunoreactivity in oligodendroglioma. (WC/jensflorian)
Demonstration of IDH1 R132H mutation in oligodendroglioma. (WC/jensflorian)
www:
- Oligodendroglioma - several images (upmc.edu).
- Oligodendroglioma with plasmacytoid cells (frontalcortex.com).
Histologic grading
Come in two flavours:
- WHO grade II.
- This is most oligodendrogliomas.
- Without genetic testing for IDH-1/2 and LOH 1p/19q, the tumor is called Oligodendroglioma, NOS.
- WHO grade III.
IHC
Features:
- MAP2 +ve.[8]
- GFAP +ve (variable).
- Some subtypes +ve - should not be used to distinguish.[9]
- EMA +ve.
- IDH-1 (R132H) +ve (approx 85%).
- ATRX +ve (nuclear retention).
- Useful for differentiating astrocytoma vs. oligodendroglioma.[12]
- H3K27me3 +ve (nuclear loss).[13]
- SOX10 +ve (up to 80%).[14]
- p53 -ve or low expressed.
- Ki-67 (usu. >5% in grade II).
- May have neuronal "islands" (Synapto +ve, NeuN +ve).
Molecular pathology
Combined losses of 1p and 19q both and presence of IDH1/2 mutation is required for final diagnosis and is prognostic and therapeutic relevant:[15][16][17]
- Greater chemosensitivity
- Better prognosis.
Note:
- Consider underdiagnosed Glioblastoma, IDH-wildtype when tumor is IDH1/2 wildtype and has no LOH 1p/19q and no ATRX loss.
- IDH-1 mutant, but non-codeleted tumors are no longer classified as oligodendrogliomas on molecular basis. These tumors are classified either as IDH-mutant astrocytoma or glioblastoma, depending on histologic features present.
See also
References
- ↑ Hartmann, C.; von Deimling, A. (2009). "Molecular pathology of oligodendroglial tumors.". Recent Results Cancer Res 171: 25-49. doi:10.1007/978-3-540-31206-2_2. PMID 19322536.
- ↑ 2.0 2.1 Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 98. ISBN 978-0443069826.
- ↑ Louis, DN.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, WK.; Ohgaki, H.; Wiestler, OD. et al. (Jun 2016). "The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary.". Acta Neuropathol 131 (6): 803-20. doi:10.1007/s00401-016-1545-1. PMID 27157931.
- ↑ 4.0 4.1 Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 94. ISBN 978-0443069826.
- ↑ URL: http://www.emedicine.com/radio/topic481.htm.
- ↑ Giannini, C.; Scheithauer, BW.; Weaver, AL.; Burger, PC.; Kros, JM.; Mork, S.; Graeber, MB.; Bauserman, S. et al. (Mar 2001). "Oligodendrogliomas: reproducibility and prognostic value of histologic diagnosis and grading.". J Neuropathol Exp Neurol 60 (3): 248-62. PMID 11245209.
- ↑ Aldape, K.; Burger, PC.; Perry, A. (Feb 2007). "Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma.". Arch Pathol Lab Med 131 (2): 242-51. doi:10.1043/1543-2165(2007)131[242:CAOQLA]2.0.CO;2. PMID 17284109.
- ↑ Suzuki SO, Kitai R, Llena J, Lee SC, Goldman JE, Shafit-Zagardo B (May 2002). "MAP-2e, a novel MAP-2 isoform, is expressed in gliomas and delineates tumor architecture and patterns of infiltration". J. Neuropathol. Exp. Neurol. 61 (5): 403–12. PMID 12025943.
- ↑ Perry, Arie; Brat, Daniel J. (2010). Practical Surgical Neuropathology: A Diagnostic Approach: A Volume in the Pattern Recognition series (1st ed.). Churchill Livingstone. pp. 98. ISBN 978-0443069826.
- ↑ Rodriguez, FJ.; Tihan, T.; Lin, D.; McDonald, W.; Nigro, J.; Feuerstein, B.; Jackson, S.; Cohen, K. et al. (Aug 2014). "Clinicopathologic features of pediatric oligodendrogliomas: a series of 50 patients.". Am J Surg Pathol 38 (8): 1058-70. doi:10.1097/PAS.0000000000000221. PMID 24805856.
- ↑ Sipayya, V.; Sharma, I.; Sharma, KC.; Singh, A.. "Immunohistochemical expression of IDH1 in gliomas: a tissue microarray-based approach.". J Cancer Res Ther 8 (4): 598-601. doi:10.4103/0973-1482.106567. PMID 23361281.
- ↑ Reuss, DE.; Sahm, F.; Schrimpf, D.; Wiestler, B.; Capper, D.; Koelsche, C.; Schweizer, L.; Korshunov, A. et al. (Jan 2015). "ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma.". Acta Neuropathol 129 (1): 133-46. doi:10.1007/s00401-014-1370-3. PMID 25427834.
- ↑ Filipski, K.; Braun, Y.; Zinke, J.; Roller, B.; Baumgarten, P.; Wagner, M.; Senft, C.; Zeiner, PS. et al. (May 2019). "Lack of H3K27 trimethylation is associated with 1p/19q codeletion in diffuse gliomas.". Acta Neuropathol. doi:10.1007/s00401-019-02025-9. PMID 31065834.
- ↑ Bannykh, SI.; Stolt, CC.; Kim, J.; Perry, A.; Wegner, M. (Jan 2006). "Oligodendroglial-specific transcriptional factor SOX10 is ubiquitously expressed in human gliomas.". J Neurooncol 76 (2): 115-27. doi:10.1007/s11060-005-5533-x. PMID 16205963.
- ↑ Fontaine D, Vandenbos F, Lebrun C, Paquis V, Frenay M (2008). "[Diagnostic and prognostic values of 1p and 19q deletions in adult gliomas: critical review of the literature and implications in daily clinical practice]" (in French). Rev. Neurol. (Paris) 164 (6-7): 595–604. doi:10.1016/j.neurol.2008.04.002. PMID 18565359.
- ↑ Wiestler, B.; Capper, D.; Hovestadt, V.; Sill, M.; Jones, DT.; Hartmann, C.; Felsberg, J.; Platten, M. et al. (Dec 2014). "Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial.". Neuro Oncol 16 (12): 1630-8. doi:10.1093/neuonc/nou138. PMID 25028501.
- ↑ Cairncross, G.; Wang, M.; Shaw, E.; Jenkins, R.; Brachman, D.; Buckner, J.; Fink, K.; Souhami, L. et al. (Jan 2013). "Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402.". J Clin Oncol 31 (3): 337-43. doi:10.1200/JCO.2012.43.2674. PMID 23071247.