Adenocarcinoma of the lung
Jump to navigation
Jump to search
Adenocarcinoma of the lung, also lung adenocarcinoma, is common malignant lung tumour.
| Adenocarcinoma of the lung | |
|---|---|
| Diagnosis in short | |
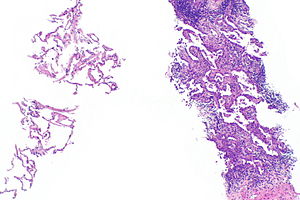 Invasive adenocarcinoma, acinar pattern (right of image) and benign lung (left of image). H&E stain. | |
|
| |
| LM | +/-nuclear atypia (may be absent in mucinous tumours), eccentrically placed nuclei, usu. abundant cytoplasm (classically with mucin vacuoles), often conspicuous nucleoli, +/-nuclear pseudoinclusions |
| LM DDx | atypical adenomatous hyperplasia of the lung, adenocarcinoma in situ, squamous cell carcinoma of the lung, small cell carcinoma of the lung, non-small cell lung carcinoma, malignant mesothelioma, metastatic adenocarcinoma (esp. colorectal adenocarcinoma, breast adenocarcinoma (invasive ductal carcinoma of the breast, invasive lobular carcinoma)) |
| IHC | CK7 +ve, TTF-1 +ve, CK20 -ve, p40 -ve, p63 -ve (usually) |
| Molecular | +/-EGFR mutations, +/-ALK chromosomal translocation (inv(2)(p21p23) -- EML4-ALK fusion) |
| Staging | lung cancer staging |
| Site | lung - see lung tumours |
|
| |
| Prevalence | most common primary lung tumour |
| Radiology | lung mass - typically peripheral lesion (distant from large airways), may be multifocal |
| Prognosis | dependent on stage (minimally invasive and noninvasive: very good; invasive: moderate) |
| Clin. DDx | other lung tumours - primary and metastatic |
| Treatment | surgical resection if feasible |
General
- Adenocarcinoma is the most common (primary lung cancer).[1]
- Adenocarcinoma is the non-smoker tumour - SCLC and squamous are more strongly associated with smoking.
- Lung adenocarcinoma is the most common brain metastasis.[2]
Treatment:
- Lung adenocarcinoma may be treated with EGFR inhibitors (e.g. gefitinib (Iressa), erlotinib (Tarceva)).[3]
Patients that receive EGFR inhibitors classically are:[4]
- Non-smokers.
- Female.
- Asian.
- Caucasians also benefit.[5]
Gross
- Classically peripheral lesions.
- May be multifocal.
Image
Microscopic
Features:
- +/-Nuclear atypia - important.
- May be absent in mucinous tumours - may look similar to foveolar epithelium.
- Eccentrically placed nuclei.
- Abundant cytoplasm - classically with mucin vacuoles.
- Often conspicuous nucleoli.
- +/-Nuclear pseudoinclusions.
Negatives:
- Lack of intercellular bridges.
Patterns:[6]
- Lepidic - tumour grows long the alveolar wall; means scaly covering.[7] At lower power, the shapes should still resemble lung acini.
- Acinar - berry-shaped glands, smaller than lung acini.
- Papillary - fibrovascular cores.
- Micropapillary - nipple shaped projections without fibrovascular cores.
- Solid - sheet of cells.
Notes:
- Lymphovascular invasion is common.
- Micropapillary predominant pattern and tumours with any amount of the lepidic pattern are associated with EGFR mutations.[8]
DDx:
- Atypical adenomatous hyperplasia of the lung - spaced hobnail cells, mild-to-moderate nuclear atypia, small lesion (< 5 mm).
- Adenocarcinoma in situ.
- Squamous cell carcinoma of the lung.
- Small cell carcinoma of the lung.
- Non-small cell lung carcinoma - diagnosis should be avoided if possible.
- Malignant mesothelioma.
- Metastatic adenocarcinoma.
- Colorectal adenocarcinoma.
- Breast adenocarcinoma.
- Other carcinomas.
- Carcinomas of the bronchial glands, e.g. adenoid cystic carcinoma.
Images
Acinar adenocarcinoma
Mucinous adenocarcinoma
Papillary adenocarcinoma
www
- BAC mucinous type adjacent to benign (pathpedia.com).
- BAC mucinous and nonmucinous (cancergrace.org).[9]
- Lepidic adenocarcinoma with invasive (flickr.com/Yale Rosen).
- Lepidic adenocarcinoma (flickr.com/Yale Rosen).
- Mucinous adenocarcinoma (pathlabmed.typepad.com).
- Non-mucinous adenocarcinoma in situ (flickr.com/Yale Rosen).
- Non-mucinous adenocarcinoma in situ (flickr.com/Yale Rosen).
- Acinar adenocarcinoma (flickr.com/Yale Rosen).
- Acinar adenocarcinoma (flickr.com/Yale Rosen).
Classification
Classification based on extent:[6]
- Adenocarcinoma in situ (AIS) - previously known as BAC.
- Subtypes: nonmucinous, mucinous, mixed mucinous/nonmucinous.
- Definition: lack of invasion into the stroma, vascular spaces and pleura.
- Must have a lepidic growth pattern.[10]
- Minimally invasive adenocarcinoma (MIA).
- Lepidic growth with up to 5 mm of invasion.
- Subtypes: nonmucinous (most common), mucinous (uncommon), mixed (mucinous/nonmucinous).
- Should not have lymphovascular invasion.[citation needed]
- Invasive adenocarcinoma:
- Subtypes: micropapillary, mucinous (previously mucinous BAC), colloid, fetal, enteric.
Grading
Graded G1-G4 - as per CAP protocol (version 3.4.0.0):[11]
- G1 = lepidic.
- G2 = acinar, papillary, cribriform.
- G3 = micropapillary, solid, mucinous, colloid.
- G4 = undifferentiated - not used for lung adenocarcinoma; it used for small cell carcinoma and large cell carcinoma.
Note:
- There is no consensus currently on grading - as per the international consensus guidelines of 2011.[6]
IHC
Primary versus metastatic:
- TTF-1 +ve.
- CK7 +ve.
- CK20 -ve.
Panel for adenocarcinoma versus SCC:
Others:
- p63 -ve -- occasionally +ve.
- Vimentin -ve/+ve (+ve relatively common).
- Poor prognosticator.[13]
Note:
- In mucinous adenocarcinoma of the lung TTF-1 is usu. -ve (46% +ve) and napsin is usu. -ve (36% +ve).
- Positive staining is unusual but useful if present, as metastatic disease is uniformily negative for both.[14]
Molecular
- EGFR mutations (typically assessed by PCR) - respond to TKIs (e.g. gefitinib, erlotinib) if:[15]
- Exon 19 deletion.
- Exon 21 L858R.
- Natural history of mutation is suspected to have a better prognosis vs. wild-type.[16]
- KRAS mutations are absent, i.e. wild-type KRAS.[17]
- ALK chromosomal translocation (inv(2)(p21p23) -- EML4-ALK fusion).[18]
- Associated with a poor prognosis.[19]
- Amenable to treatment with TKI.
- See lung carcinoma with ALK rearrangement.
- Do not occur with EGRF mutations or KRAS mutations.[20]
Sign out
Biopsy
Consensus recommendations:[6]
- Adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma should not be used in the reporting of small biopsies and cytology.
- Tumours with a non-invasive pattern are referred to by their pattern, e.g. lepidic growth, not as AIS.
Lung, Right Upper Lobe, Core Biopsy: - INVASIVE ADENOCARCINOMA, NON-MUCINOUS. Comment: The adenocarcinoma is positive for TTF-1 and napsin. EGFR/ALK testing was ordered.
Mucinous adenocarcinoma with noncontributory stains
Lung, Right Upper Lobe, Core Biopsy: - ADENOCARCINOMA, MUCINOUS, see comment. Comment: The adenocarcinoma is negative for both napsin and TTF-1. EGFR/ALK testing was ordered. The findings are compatible with a primary or secondary adenocarcinoma; clinical and radiologic correlation is required.
Block letters
LUNG, LEFT, BIOPSY: - ADENOCARCINOMA, LEPIDIC GROWTH; INVASION CANNOT BE EXCLUDED IN THIS SMALL SPECIMEN.
LUNG, RIGHT UPPER LOBE, NEEDLE BIOPSY: - INVASIVE ADENOCARCINOMA, NON-MUCINOUS. COMMENT: The tumour stains as follows: POSITIVE: TTF-1. NEGATIVE: p40. The immunoprofile is compatible with lung adenocarcinoma.
MASS, LEFT LOWER LOBE OF LUNG, BIOPSY: - INVASIVE ADENOCARCINOMA. COMMENT: The tumour is positive for TTF-1. Tissue will be sent for molecular testing and the results reported as an addendum.
Resection
LUNG, LEFT UPPER LOBE, LOBECTOMY: - ADENOCARCINOMA WITH AN ACINAR PATTERN, SOLID PATTERN, MICROPAPILLARY PATTERN AND LEPIDIC PATTERN -- PATTERNS IN ORDER OF PREVALENCE. - MARGINS NEGATIVE FOR MALIGNANCY. - THREE LYMPH NODES NEGATIVE FOR MALIGNANCY (0 POSITIVE/3). - PLEASE SEE TUMOUR SUMMARY.
LUNG, RIGHT UPPER LOBE, LOBECTOMY: - MULTIPLE ADENOCARCINOMAS (x2) WITH AN ACINAR PATTERN, SOLID PATTERN, MICROPAPILLARY PATTERN AND LEPIDIC PATTERN -- PATTERNS IN ORDER OF PREVALENCE. - MARGINS NEGATIVE FOR MALIGNANCY. - FOUR LYMPH NODES NEGATIVE FOR MALIGNANCY (0 POSITIVE/4). - LYMPHOVASCULAR INVASION PRESENT. - PLEASE SEE TUMOUR SUMMARY AND COMMENT. COMMENT: The histology of the two adenocarcinomas resemble one another and lymphovascular invasion is present. These findings favour that the smaller tumor is a metastasis, rather than a synchronous primary.
Micro
Size (tissue): scant tissue (<0.5 cm). Gland formation: focal, poorly formed. Cell size: large. Cytoplasm: moderate-to-abundant, grey-eosinophilic. Nucleus location: eccentric. Nuclear pleomorphism: moderate. Nuclear moulding: absent. Nucleoli: present, prominent. Nuclear pseudoinclusions: present.
Number of cores: 3. Length of cores (total): 2.0 cm. Gland formation: present. Cell size: large. Cytoplasm: moderate, grey-eosinophilic. Necrosis: none apparent. Mucin: none. Nucleus location: eccentric. Nuclear pleomorphism: moderate. Nuclear moulding: absent. Nuclear pseudoinclusions: absent. Nuclear shape/arrangment: cigar-like/pseudostratified. Nucleoli: present.
Mucinous
The sections show cores with well-formed glands composed of foveolar-like columnar cells with a relatively bland cytomorphology. Mitotic activity is not readily apparent. A small amount of non-lesional lung parenchyma is present.
Lung cancer staging
Main article: Lung cancer staging
See also
References
- ↑ Lutschg JH (January 2009). "Lung cancer". N. Engl. J. Med. 360 (1): 87-8; author reply 88. doi:10.1056/NEJMc082208. PMID 19118313.
- ↑ Nayak, L.; Lee, EQ.; Wen, PY. (Feb 2012). "Epidemiology of brain metastases.". Curr Oncol Rep 14 (1): 48-54. doi:10.1007/s11912-011-0203-y. PMID 22012633.
- ↑ Sun Y, Ren Y, Fang Z, et al. (October 2010). "Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases". J. Clin. Oncol. 28 (30): 4616–20. doi:10.1200/JCO.2010.29.6038. PMID 20855837.
- ↑ Job B, Bernheim A, Beau-Faller M, et al. (2010). "Genomic Aberrations in Lung Adenocarcinoma in Never Smokers". PLoS One 5 (12): e15145. doi:10.1371/journal.pone.0015145. PMC 2997777. PMID 21151896. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2997777/.
- ↑ Rosell, R.; Moran, T.; Cardenal, F.; Porta, R.; Viteri, S.; Molina, MA.; Benlloch, S.; Taron, M. (Oct 2010). "Predictive biomarkers in the management of EGFR mutant lung cancer.". Ann N Y Acad Sci 1210: 45-52. doi:10.1111/j.1749-6632.2010.05775.x. PMID 20973798.
- ↑ 6.0 6.1 6.2 6.3 Travis WD, Brambilla E, Noguchi M, et al. (February 2011). "International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma". J Thorac Oncol 6 (2): 244–85. doi:10.1097/JTO.0b013e318206a221. PMID 21252716.
- ↑ URL: http://medical-dictionary.thefreedictionary.com/lepidic. Accessed on: 8 August 2013.
- ↑ Shim, HS.; Lee, da H.; Park, EJ.; Kim, SH. (Oct 2011). "Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification.". Arch Pathol Lab Med 135 (10): 1329-34. doi:10.5858/arpa.2010-0493-OA. PMID 21970488.
- ↑ URL: http://cancergrace.org/lung/2007/05/14/bac-mucinous-and-non-mucinous/. Accessed on: 8 August 2013.
- ↑ Borczuk, AC. (Jan 2012). "Assessment of invasion in lung adenocarcinoma classification, including adenocarcinoma in situ and minimally invasive adenocarcinoma.". Mod Pathol 25 Suppl 1: S1-10. doi:10.1038/modpathol.2011.151. PMID 22214965.
- ↑ CAP Lung protocol. Version: 3.4.0.0. URL: http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp-lung-16protocol-3400.pdf. Accessed on: March 23, 2016.
- ↑ Bishop, JA.; Teruya-Feldstein, J.; Westra, WH.; Pelosi, G.; Travis, WD.; Rekhtman, N. (Mar 2012). "p40 (ΔNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma.". Mod Pathol 25 (3): 405-15. doi:10.1038/modpathol.2011.173. PMID 22056955.
- ↑ Dauphin, M.; Barbe, C.; Lemaire, S.; Nawrocki-Raby, B.; Lagonotte, E.; Delepine, G.; Birembaut, P.; Gilles, C. et al. (Jul 2013). "Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas.". Lung Cancer 81 (1): 117-22. doi:10.1016/j.lungcan.2013.03.011. PMID 23562674.
- ↑ Rossi, G.; Cavazza, A.; Righi, L.; Sartori, G.; Bisagni, A.; Longo, L.; Pelosi, G.; Papotti, M. (Aug 2014). "Napsin-A, TTF-1, EGFR, and ALK Status Determination in Lung Primary and Metastatic Mucin-Producing Adenocarcinomas.". Int J Surg Pathol 22 (5): 401-7. doi:10.1177/1066896914527609. PMID 24651909.
- ↑ John, T.; Liu, G.; Tsao, MS. (Aug 2009). "Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors.". Oncogene 28 Suppl 1: S14-23. doi:10.1038/onc.2009.197. PMID 19680292.
- ↑ URL: http://www.mycancergenome.org/mutation.php?dz=nsclc&gene=egfr&code=l858r. Accessed on: 27 April 2012.
- ↑ Pao, W.; Wang, TY.; Riely, GJ.; Miller, VA.; Pan, Q.; Ladanyi, M.; Zakowski, MF.; Heelan, RT. et al. (Jan 2005). "KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib.". PLoS Med 2 (1): e17. doi:10.1371/journal.pmed.0020017. PMID 15696205.
- ↑ Li, Y.; Ye, X.; Liu, J.; Zha, J.; Pei, L. (Jan 2011). "Evaluation of EML4-ALK fusion proteins in non-small cell lung cancer using small molecule inhibitors.". Neoplasia 13 (1): 1-11. PMID 21245935.
- ↑ Yang, P.; Kulig, K.; Boland, JM.; Erickson-Johnson, MR.; Oliveira, AM.; Wampfler, J.; Jatoi, A.; Deschamps, C. et al. (Jan 2012). "Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma.". J Thorac Oncol 7 (1): 90-7. doi:10.1097/JTO.0b013e31823c5c32. PMID 22134072.
- ↑ Gainor, JF.; Varghese, AM.; Ou, SH.; Kabraji, S.; Awad, MM.; Katayama, R.; Pawlak, A.; Mino-Kenudson, M. et al. (Aug 2013). "ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer.". Clin Cancer Res 19 (15): 4273-81. doi:10.1158/1078-0432.CCR-13-0318. PMID 23729361.