Difference between revisions of "Programmed death-ligand 1"
Jump to navigation
Jump to search
| (15 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
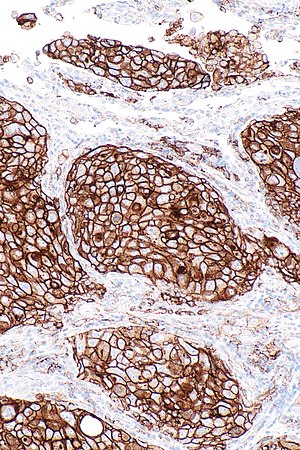
'''Programmed death-ligand 1''', commonly abbreviated '''PD-L1''', is a protein with an important role in | [[Image:PD-L1_positive_lung_adenocarcinoma_--_intermed_mag.jpg|right|thumb|[[Micrograph]] showing a PD-L1 positive non-small cell lung carcinoma (NSCLC). PD-L1 [[immunostain]] (22C3). (WC)]] | ||
'''Programmed death-ligand 1''', commonly abbreviated '''PD-L1''', is a protein with an important role in immune system regulation and [[cancer]]. | |||
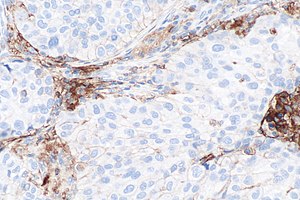
[[Image:PD-L1 negative lung adenocarcinoma -- high mag.jpg|right|thumb|[[Micrograph]] showing a PD-L1 negative [[NSCLC]]. PD-L1 immunostain (22C3). (WC)]] | |||
Normally, PD-L1 on cells binds with [[programmed cell death 1]] on the T lymphocytes.<ref name=pmid22658126/> | Normally, PD-L1 on cells binds with [[programmed cell death 1]] on the T lymphocytes.<ref name=pmid22658126/> | ||
| Line 6: | Line 8: | ||
==General== | ==General== | ||
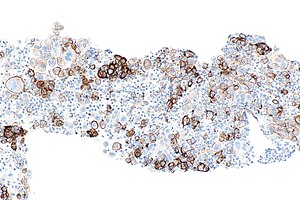
[[Image:PD-L1_positive_lung_adenocarcinoma_in_lymph_node_--_intermed_mag.jpg|thumb|right|PD-L1 positive lung adenocarcinoma in a lymph node. 22C3 PD-L1 immunostain. (WC)]] | |||
*In theory, positive PD-L1 [[IHC|immunostaining]] predicts response to anti-PD-L1 drugs.<ref name=pmid26970723/> | |||
**Carcinoma cell is considered "PD-L1 positive" if the cell membrane is partially or completely stained.<ref name="pmid27389313">{{Cite journal | last1 = Scheel | first1 = AH. | last2 = Dietel | first2 = M. | last3 = Heukamp | first3 = LC. | last4 = Jöhrens | first4 = K. | last5 = Kirchner | first5 = T. | last6 = Reu | first6 = S. | last7 = Rüschoff | first7 = J. | last8 = Schildhaus | first8 = HU. | last9 = Schirmacher | first9 = P. | title = Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. | journal = Mod Pathol | volume = 29 | issue = 10 | pages = 1165-72 | month = Oct | year = 2016 | doi = 10.1038/modpathol.2016.117 | PMID = 27389313 }}</ref> | |||
*It is, however, more complex than that. Some tumour types are invariably positive, e.g. classical Hodgkin lymphoma, so testing is unhelpful. In contrast, tumors such as malignant melanoma respond regardless of PD-L1 immunoexpression. | |||
*The plethora of companion diagnostics developed for each PD-1/ PD-L1 inhibitor has created challenges, as these assays include different IHC antibody clones, staining protocols and platforms, scoring systems, and cutoffs for defining positivity. | *The plethora of companion diagnostics developed for each PD-1/ PD-L1 inhibitor has created challenges, as these assays include different IHC antibody clones, staining protocols and platforms, scoring systems, and cutoffs for defining positivity. | ||
**Nivolumab - 28-8 (Dako) | |||
**Pembrolizumab - 22C3 (Dako) | |||
**Aterolizumab - SP142 (Ventana) | |||
**Durvalumab - SP263 (Ventana) | |||
**Avelumab - 73-10 (Dako) | |||
===Background=== | ===Background=== | ||
| Line 24: | Line 34: | ||
| [[PD-L1]] (CD274), PD-L2 (CD273) | | [[PD-L1]] (CD274), PD-L2 (CD273) | ||
|} | |} | ||
===Adequacy of PD-L1=== | |||
*100 cells or more.<ref name=pmid31097091>{{cite journal |authors=Wang H, Agulnik J, Kasymjanova G, Fiset PO, Camilleri-Broet S, Redpath M, Cohen V, Small D, Pepe C, Sakr L, Spatz A |title=The metastatic site does not influence PD-L1 expression in advanced non-small cell lung carcinoma |journal=Lung Cancer |volume=132 |issue= |pages=36–38 |date=June 2019 |pmid=31097091 |doi=10.1016/j.lungcan.2019.04.009 |url=}}</ref> | |||
==Prognosis== | ==Prognosis== | ||
| Line 30: | Line 42: | ||
==Drugs - Immune checkpoint inhibitors== | ==Drugs - Immune checkpoint inhibitors== | ||
*PD-1 inhibitors: | *PD-1 inhibitors: | ||
**Nivolumab | **Nivolumab (''Opdivo'', Bristol-Myers Squibb). | ||
**Pembrolizumab | **Pembrolizumab (''Keytruda'', Merck). | ||
*PD-L1 inhibitors: | *PD-L1 inhibitors: | ||
**Atezolizumab.<ref name=pmid26970723>{{Cite journal | last1 = Fehrenbacher | first1 = L. | last2 = Spira | first2 = A. | last3 = Ballinger | first3 = M. | last4 = Kowanetz | first4 = M. | last5 = Vansteenkiste | first5 = J. | last6 = Mazieres | first6 = J. | last7 = Park | first7 = K. | last8 = Smith | first8 = D. | last9 = Artal-Cortes | first9 = A. | title = Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. | journal = Lancet | volume = | issue = | pages = | month = Mar | year = 2016 | doi = 10.1016/S0140-6736(16)00587-0 | PMID = 26970723 }}</ref> | **Atezolizumab (''Tecentriq'', Roche).<ref name=pmid26970723>{{Cite journal | last1 = Fehrenbacher | first1 = L. | last2 = Spira | first2 = A. | last3 = Ballinger | first3 = M. | last4 = Kowanetz | first4 = M. | last5 = Vansteenkiste | first5 = J. | last6 = Mazieres | first6 = J. | last7 = Park | first7 = K. | last8 = Smith | first8 = D. | last9 = Artal-Cortes | first9 = A. | title = Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. | journal = Lancet | volume = | issue = | pages = | month = Mar | year = 2016 | doi = 10.1016/S0140-6736(16)00587-0 | PMID = 26970723 }}</ref> | ||
**Durvalumab. | **Durvalumab (''Imfinzi'', AstraZeneca). | ||
**Avelumab | **Avelumab (''Bavencio'', Merck/Pfizer). | ||
===Anti-PD-L1 drugs - use=== | ===Anti-PD-L1 drugs - use=== | ||
| Line 46: | Line 57: | ||
*[[Renal cell carcinoma]]. | *[[Renal cell carcinoma]]. | ||
*[[Urothelial carcinoma]]. | *[[Urothelial carcinoma]]. | ||
*[[Merkel cell carcinoma]] | |||
*[[Acute myeloid leukemia]] | |||
==See also== | ==See also== | ||
Latest revision as of 19:59, 22 August 2024

Micrograph showing a PD-L1 positive non-small cell lung carcinoma (NSCLC). PD-L1 immunostain (22C3). (WC)
Programmed death-ligand 1, commonly abbreviated PD-L1, is a protein with an important role in immune system regulation and cancer.
Normally, PD-L1 on cells binds with programmed cell death 1 on the T lymphocytes.[1]
PD-L1 is also known as CD274.[2]
General
- In theory, positive PD-L1 immunostaining predicts response to anti-PD-L1 drugs.[3]
- Carcinoma cell is considered "PD-L1 positive" if the cell membrane is partially or completely stained.[4]
- It is, however, more complex than that. Some tumour types are invariably positive, e.g. classical Hodgkin lymphoma, so testing is unhelpful. In contrast, tumors such as malignant melanoma respond regardless of PD-L1 immunoexpression.
- The plethora of companion diagnostics developed for each PD-1/ PD-L1 inhibitor has created challenges, as these assays include different IHC antibody clones, staining protocols and platforms, scoring systems, and cutoffs for defining positivity.
- Nivolumab - 28-8 (Dako)
- Pembrolizumab - 22C3 (Dako)
- Aterolizumab - SP142 (Ventana)
- Durvalumab - SP263 (Ventana)
- Avelumab - 73-10 (Dako)
Background
Cytotoxic T cell function is regulated by receptor pairs found on the tumour and lymphocyte:[1]
| Function | Tumour cell | T cell |
|---|---|---|
| Antigen presentation | MHC | TCR |
| Signal inhibition | PD-1 | PD-L1 (CD274), PD-L2 (CD273) |
Adequacy of PD-L1
- 100 cells or more.[5]
Prognosis
- Good prognosis - in high-grade ovarian serous carcinoma, associated with tumour-infiltrating lymphocytes.[6]
Drugs - Immune checkpoint inhibitors
- PD-1 inhibitors:
- Nivolumab (Opdivo, Bristol-Myers Squibb).
- Pembrolizumab (Keytruda, Merck).
- PD-L1 inhibitors:
- Atezolizumab (Tecentriq, Roche).[3]
- Durvalumab (Imfinzi, AstraZeneca).
- Avelumab (Bavencio, Merck/Pfizer).
Anti-PD-L1 drugs - use
PD-L1 antibodies are being used to treat:[7]
- Malignant melanoma.
- Non-small cell lung cancer.
- Associated with response predicted by tumour-infiltrating lymphocytes and PD-L1 IHC positivity of the tumour cells.[3]
- Renal cell carcinoma.
- Urothelial carcinoma.
- Merkel cell carcinoma
- Acute myeloid leukemia
See also
References
- ↑ 1.0 1.1 Ribas, A. (Jun 2012). "Tumor immunotherapy directed at PD-1.". N Engl J Med 366 (26): 2517-9. doi:10.1056/NEJMe1205943. PMID 22658126.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 605402
- ↑ 3.0 3.1 3.2 Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D. et al. (Mar 2016). "Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial.". Lancet. doi:10.1016/S0140-6736(16)00587-0. PMID 26970723.
- ↑ Scheel, AH.; Dietel, M.; Heukamp, LC.; Jöhrens, K.; Kirchner, T.; Reu, S.; Rüschoff, J.; Schildhaus, HU. et al. (Oct 2016). "Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas.". Mod Pathol 29 (10): 1165-72. doi:10.1038/modpathol.2016.117. PMID 27389313.
- ↑ Wang H, Agulnik J, Kasymjanova G, Fiset PO, Camilleri-Broet S, Redpath M, Cohen V, Small D, Pepe C, Sakr L, Spatz A (June 2019). "The metastatic site does not influence PD-L1 expression in advanced non-small cell lung carcinoma". Lung Cancer 132: 36–38. doi:10.1016/j.lungcan.2019.04.009. PMID 31097091.
- ↑ Webb, JR.; Milne, K.; Kroeger, DR.; Nelson, BH. (May 2016). "PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer.". Gynecol Oncol 141 (2): 293-302. doi:10.1016/j.ygyno.2016.03.008. PMID 26972336.
- ↑ Gandini, S.; Massi, D.; Mandalà, M. (Apr 2016). "PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis.". Crit Rev Oncol Hematol 100: 88-98. doi:10.1016/j.critrevonc.2016.02.001. PMID 26895815.