Difference between revisions of "Liver neoplasms"
(→Hepatic adenoma: split out) |
|||
| Line 307: | Line 307: | ||
*[[Cholangiocarcinoma]]. | *[[Cholangiocarcinoma]]. | ||
*[[Hepatocellular carcinoma]], pseudoglandular.<ref name=pmid2440554>{{Cite journal | last1 = Kondo | first1 = Y. | last2 = Nakajima | first2 = T. | title = Pseudoglandular hepatocellular carcinoma. A morphogenetic study. | journal = Cancer | volume = 60 | issue = 5 | pages = 1032-7 | month = Sep | year = 1987 | doi = | PMID = 2440554 }}</ref> | *[[Hepatocellular carcinoma]], pseudoglandular.<ref name=pmid2440554>{{Cite journal | last1 = Kondo | first1 = Y. | last2 = Nakajima | first2 = T. | title = Pseudoglandular hepatocellular carcinoma. A morphogenetic study. | journal = Cancer | volume = 60 | issue = 5 | pages = 1032-7 | month = Sep | year = 1987 | doi = | PMID = 2440554 }}</ref> | ||
*[[Epithelioid hemangioendothelioma]]. (???) | |||
====Image==== | ====Image==== | ||
Revision as of 16:48, 5 September 2014
This article examines liver neoplasms and pre-malignant lesions of the liver. In North America, most malignant liver lesions are metastases.
This article focuses on primary malignancies of the liver, neoplastic liver lesions, and biliary malignancies. It only briefly discusses metastatic lesions. An introduction to liver pathology is in the liver article. Medical liver disease is dealt with in the medical liver disease article.
Overview
Dysplasic lesions of the liver
Types:[1]
- "Large cell dysplasia" (AKA large cell change) - not considered a precursor for HCC, not considered a dysplasia.[2]
- Small liver cell dysplasia (AKA small cell dysplasia).
- Low grade dysplasia.
- High grade dysplasia.
Neoplastic lesions
Malignant lesions of the liver
- Hepatocellular carcinoma (HCC) - most common malignant liver primary in adults.
- Hepatoblastoma - malignant liver primary in children.
- Intrahepatic cholangiocarcinoma (ICC).[3]
- Combined hepatocellular carcinoma and intrahepatic cholangiocarcinoma (CHC).
Lesions that arise in a non-cirrhotic liver
Hepatocellular:
Other:
Tabular comparison
Precursors
Features of HCC & its precursors - generated from DCHH[4] and STC:
| Features | SLCD | Low-grade dysplasia | High-grade dysplasia | HCC |
| Plate thickness | <3 cells | <=2 cells | <=3 cells, usu. >2 cells | >3 cells |
| Reticulin (stain) | intact chicken wire | intact chicken wire | intact chicken wire | damaged chicken wire |
| Nuclear changes | nuc. enlargement, hyperchromasia |
+/- atypia (???) | marked atypia | +/- incr. NCR, +/-irreg. nuc. contour |
| Cytoplasmic change | hyperchromasia, decr. as cell size preserved |
none (???) | +/- basophilia | variable (lighter vs. hyperchromasia) |
| Portal tracts | ? | loss of portal tracts | loss of portal tracts | loss of portal tracts |
| Management | follow ??? | follow | ablate | ablate/surgery |
Abbreviations:
- SLCD = small liver cell dysplasia.
Notes:
- Large cell dysplasia:
- Cell size ~ 2x normal, NC ratio ~ normal.
- SLCD:
- Cell size ~ 1/2x normal, NC ratio - increased.
Hepatic tumours
Benign:
| Entity | Gross | Microscopic | IHC/stains | Other | Images |
|---|---|---|---|---|---|
| Hepatic hemangioma | similar to normal liver parenchyma, red (hemorrhagic), well-circumscribed | spaces lined by benign endothelial cells | CD31+ (???) | - | gross (rsna.org) |
| Focal nodular hyperplasia | central scar, large vessels, usu. well-circumscribed | large arteries, unpaired arteries, bile duct proliferation | usu. diagnosed by imaging | gross (rsna.org) | |
| Hepatocellular adenoma | subcapsular, well-circumscribed | loss of portal tracts, nuclear glycogenation | reticulin - liver plate thickness <= 3 | background not cirrhotic, assoc. OCP | gross (mda-sy.com)[5] |
Malignant:
| Entity | Gross | Microscopic | IHC/stains | Other | Images |
|---|---|---|---|---|---|
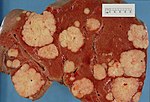
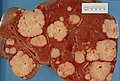
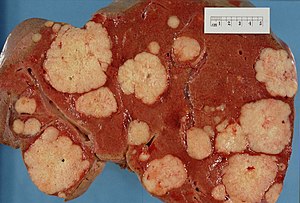
| Liver metastasis | multiple, white lesions | variable, usu. tubular (glandular) with pseudostratified hyperchromatic nuclei | CK7-, CK20-, HepPar-1-, CK19- | colorectal carcinoma most common | |
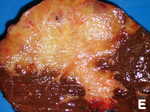
| Hepatocellular carcinoma | poorly circumscribed, +/-necrosis, +/-hemorrhage | loss of portal tracts, unpaired arteries, +/-nuclear atypia | reticulin - liver plate thickness > 3 | background often cirrhotic | |
| Cholangiocarcinoma | cauliflower-like outline, white, classically solitary, no cirrhosis | tubular architecture and mild nuclear atypia (adenocarcinoma), desmoplastic stroma | CK7+, CK19+ | background usu. not cirrhotic |
Dysplasia of the liver
Small liver cell dysplasia
- Abbreviated SLCD.
- AKA small cell dysplasia.
General
- Considered a precursor to HCC.
- Frequently found in livers with HCC - when compared to livers without HCC.[6]
Microscopic
Features:[7]
- Cells similar in size to normal hepatocytes.
- Name derived from the fact that there is also an entity that was called large cell dysplasia (AKA large liver cell dysplasia,[6] and large cell change).
- Increased NC ratio - "more blue".
- Mild nuclear and cytoplasmic hyperchromatism.
Notes:
- Normal hepatic architecture (main differentiator from HCC).
- Remember "... blue is bad".
Micrograph:
Low-grade hepatocellular dysplasia
- Generally referred to as low-grade dysplasia as the context is usually clear.
Microscopic
- Uniform cells - "noticeably different from normal".[8]
- Changes in nuclear size, irregular nuclear contour and/or changes in cytoplasm staining.
- Loss of portal tracts.
- Irregular margin.
Notes:
- DCHH describes LGD as: "normal hepatocytes in plates [of normal thickness]".[4]
DDx:
- Nodular regenerative hyperplasia - lacks: compressed rim of cells, central portal tract.[4]
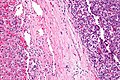
High-grade hepatocellular dysplasia
- Generally referred to as high-grade dysplasia as the context is usually clear.
General
- "Bader" version of low-grade dyplasia.
Microscopic
Features - in addition to those of low grade dysplasia:[4]
- Liver plate >2 cells thick.
- Significant nuclear atypia.
- Basophilic cytoplasm.
DDx:
Image:
Benign hepatic neoplasms
Bile duct adenoma
- Should not be confused with bile duct hamartoma.
Hepatic adenoma
- AKA hepatocellular adenoma, abbreviated HCA.
Hepatobiliary mucinous cystadenoma
- AKA biliary cystadenoma.
General
- Benign neoplasm.
- May transform into a malignancy.[9]
Microscopic
Features:
- Cystic spaces lined by a mucinous epithelium (simple columnar epithelium with a clear cytoplasm).
Note:
- Similar to pancreatic mucinous cystadenoma.
Malignant hepatic neoplasms
In North America, the most common malignant liver tumour is metastases.
Hepatoblastoma
General
- Most common liver cancer in children.[10][11]
- Rare in adolescents and adults.
- Age of diagnosis usu. ~1 year old; most less than 3 years old.
- Surgical biopsy; core needle biopsy not done as as lesion is vascular.
Associations:
Clinical:
- Usually present with hepatomegaly.
- High AFP.[13]
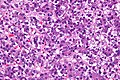
Microscopic
Features:
- Small round cell tumour.
- Fetal hepatocytes ~ 1:3 NC ratio, eosinophilic cytoplasm.
- +/-Mesenchymal component
- Immature fibrous tissue, osteoid or cartilage.
DDx:
- Small round cell tumours.
- Teratoma.
- Hepatocellular carcinoma - separated based on histomorphology alone.
Images
Subtypes
- Six histologic subtypes - that are subdivided into two groups:[14]
- Epithelial type:
- Fetal pattern.
- Embryonal and fetal pattern.
- Macrotrabecular pattern.
- May mimic hepatocellular carcinoma histologically.[15]
- Small cell undifferentiated pattern.
- Poor prognosis.
- Mixed epithelial and mesenchymal type:
- With teratoid features.
- Without teratoid features.
- Epithelial type:
IHC
- Alpha-fetoprotein +ve.
- Hepatocyte specific antigen +ve esp. in fetal component.[16]
- Beta-catenin +ve (cytoplasmic and nuclear).[16]
Hepatocellular carcinoma
- Abbreviated HCC.
Cholangiocarcinoma
Hepatic angiosarcoma
- AKA angiosarcoma of the liver.
General
- Liver angiosarcomas are associated with vinyl chloride exposure.[18]
Microscopic
Features:
- Atypical endothelial cells - may be subtle.
Hepatic metastasis
General
- Metastases are very common - often from the gastrointestinal tract, e.g. colorectal cancer.
- Most liver masses in are not biopsied... as a primary lesion is evident.[19]
- Dependent on the extent of disease, CRC metastatic to the liver may be curable with a liver resection.
- Peritoneal disease, i.e. a malignant peritoneal nodule, in the context of liver metastases does poorly, and is considered a contraindication to liver resection.[20]
- It is important to consider germ cell tumours in the DDx as these may be curable with chemotherapy.
- Clear cell variant of HCC may be misdiagnosed as metastatic clear cell carcinoma.
- Interhepatic cholangiocarcinoma is an adenocarcinoma - it may look like a metastatic lesion.
Further reading:
- Anders, RA.; Kamel, IR. (May 2007). "Biopsy considerations in the diagnosis of hepatic masses.". Clin Gastroenterol Hepatol 5 (5): 541-4. doi:10.1016/j.cgh.2007.02.028. PMID 17478344.
Gross pathology/radiology
- Multifocal or solitary.
- Classically multifocal.
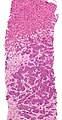
Microscopic
Features:
- Histologic features are dependent on primary and degree of differentiation.
The classic liver metastasis (colorectal carcinoma):
- Gland forming columnar shaped cells with pseudostratified hyperchromatic cigar-shaped nuclei.
DDx:
- Cholangiocarcinoma.
- Hepatocellular carcinoma, pseudoglandular.[21]
- Epithelioid hemangioendothelioma. (???)
Image
IHC
- Metastases are typically negative for HepPar-1.
- HepPar-1 (hepatocytes paraffin antibody 1) - labels hepatocellular mitochondria.[22]
Note:
- If a primary is already established by pathology and the clinical impression is a metastasis, it isn't necessary to do IHC if the morphology of the lesion in the liver is compatible with the established primary.
Sign out
LIVER, PORTION OF SEGMENTS 2 AND 3, RESECTION: - METASTATIC ADENOCARCINOMA. -- RESECTION MARGIN CLEARANCE 2 MM. - LIVER STEATOSIS, MILD.
Micro
The section show liver parenchyma with an invasive adenocarcinoma. The adenocarcinoma has well formed glands with dirty necrosis. The nuclei are appear crowded and have an ellipsoid shape. Focally, zones of necrosis are present. See background liver.
BACKGROUND LIVER (BASED ON H&E ONLY)
Fibrosis: not identified.
Fibrous septa: absent.
Septa with curved contours: absent.
Large droplet steatosis (% of hepatocytes): mild (20%).
Ballooning of hepatocytes: not identified.
Mallory-Denk bodies: not identified.
Portal inflammation: present, mild.
Interface activity: not identified.
Lobular necroinflammation: not identified.
Ducts: present in normal numbers.
Duct injury: not identified.
Ductular reaction: not identified.
Cholestasis: present peritumoural, otherwise absent.
Terminal hepatic venules: present.
Ground glass cells with routine stains: not identified.
See also
References
- ↑ STC. S.30-37, 19 Jan 2009.
- ↑ Park, YN.; Roncalli, M. (Nov 2006). "Large liver cell dysplasia: a controversial entity.". J Hepatol 45 (5): 734-43. doi:10.1016/j.jhep.2006.08.002. PMID 16982109.
- ↑ Shirakawa, H.; Kuronuma, T.; Nishimura, Y.; Hasebe, T.; Nakano, M.; Gotohda, N.; Takahashi, S.; Nakagohri, T. et al. (Mar 2009). "Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer.". Int J Oncol 34 (3): 649-56. PMID 19212669. http://www.spandidos-publications.com/serveFile/ijo_34_3_649_PDF.pdf?type=article&article_id=ijo_34_3_649&item=PDF.
- ↑ 4.0 4.1 4.2 4.3 Tadrous, Paul.J. Diagnostic Criteria Handbook in Histopathology: A Surgical Pathology Vade Mecum (1st ed.). Wiley. pp. 170-1. ISBN 978-0470519035.
- ↑ URL: http://www.mda-sy.com/vb/showthread.php?p=5083&langid=1. Accessed on: 16 February 2012.
- ↑ 6.0 6.1 Szczepański, W. (1997). "Liver cell dysplasia in liver cirrhosis and hepatocellular carcinoma.". Pol J Pathol 48 (3): 147-57. PMID 9401407.
- ↑ STC S.32, 19 Jan 2009.
- ↑ STC - 19 Jan 2009. (???)
- ↑ Yu, J.; Wang, Y.; Yu, X.; Liang, P.. "Hepatobiliary mucinous cystadenoma and cystadenocarcinoma: report of six cases and review of the literature.". Hepatogastroenterology 57 (99-100): 451-5. PMID 20698207.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 923. ISBN 0-7216-0187-1.
- ↑ URL: http://emedicine.medscape.com/article/986802-overview. Accessed on: 29 November 2009.
- ↑ DeBaun MR, Tucker MA (March 1998). "Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry". J. Pediatr. 132 (3 Pt 1): 398–400. PMID 9544889.
- ↑ URL: http://emedicine.medscape.com/article/986802-diagnosis. Accessed on: 11 February 2011.
- ↑ URL: http://emedicine.medscape.com/article/986802-diagnosis. Accessed on: 11 February 2011.
- ↑ URL: http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt{actionForm.contentReference}=cap_foundation%2FcaseOfMonth%2FMar10%2Fmar_2010_cotm_diagnosis.html&_state=maximized&_pageLabel=cntvwr#null. Accessed on: 11 February 2011.
- ↑ 16.0 16.1 Halász, J.; Holczbauer, A.; Páska, C.; Kovács, M.; Benyó, G.; Verebély, T.; Schaff, Z.; Kiss, A. (May 2006). "Claudin-1 and claudin-2 differentiate fetal and embryonal components in human hepatoblastoma.". Hum Pathol 37 (5): 555-61. doi:10.1016/j.humpath.2005.12.015. PMID 16647953.
- ↑ URL: http://www.cancer.org/cancer/bileductcancer/detailedguide/bile-duct-cancer-what-is-bile-duct-cancer. Access on: 23 May 2013.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 212. ISBN 978-1416054542.
- ↑ OA. 29 November 2009.
- ↑ Elias, D.; Rougier, P.; Mankarios, H.; Fahrat, F.; Lasser, P. (Mar 1993). "[Resectable liver metastases and synchronous extra-hepatic sites of colorectal origin. Surgical indications].". Presse Med 22 (11): 515-20. PMID 8511077.
- ↑ Kondo, Y.; Nakajima, T. (Sep 1987). "Pseudoglandular hepatocellular carcinoma. A morphogenetic study.". Cancer 60 (5): 1032-7. PMID 2440554.
- ↑ The diagnostic value of hepatocyte paraffin antibody 1 in differentiating hepatocellular neoplasms from nonhepatic tumors: a review. Lamps LW, Folpe AL. Adv Anat Pathol. 2003 Jan;10(1):39-43. Review. PMID 12502967.