Difference between revisions of "Intraductal carcinoma of the prostate"
Jump to navigation
Jump to search
| Line 123: | Line 123: | ||
==Molecular== | ==Molecular== | ||
*+/-BRCA2 mutation.<ref name=pmid28067867>{{cite journal |authors=Taylor RA, Fraser M, Livingstone J, Espiritu SM, Thorne H, Huang V, Lo W, Shiah YJ, Yamaguchi TN, Sliwinski A, Horsburgh S, Meng A, Heisler LE, Yu N, Yousif F, Papargiris M, Lawrence MG, Timms L, Murphy DG, Frydenberg M, Hopkins JF, Bolton D, Clouston D, McPherson JD, van der Kwast T, Boutros PC, Risbridger GP, Bristow RG |title=Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories |journal=Nat Commun |volume=8 |issue= |pages=13671 |date=January 2017 |pmid=28067867 |pmc=5227331 |doi=10.1038/ncomms13671 |url=}}</ref> | *+/-[[BRCA2 mutation]].<ref name=pmid28067867>{{cite journal |authors=Taylor RA, Fraser M, Livingstone J, Espiritu SM, Thorne H, Huang V, Lo W, Shiah YJ, Yamaguchi TN, Sliwinski A, Horsburgh S, Meng A, Heisler LE, Yu N, Yousif F, Papargiris M, Lawrence MG, Timms L, Murphy DG, Frydenberg M, Hopkins JF, Bolton D, Clouston D, McPherson JD, van der Kwast T, Boutros PC, Risbridger GP, Bristow RG |title=Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories |journal=Nat Commun |volume=8 |issue= |pages=13671 |date=January 2017 |pmid=28067867 |pmc=5227331 |doi=10.1038/ncomms13671 |url=}}</ref> | ||
==See also== | ==See also== | ||
Revision as of 18:26, 26 April 2021
| Intraductal carcinoma of the prostate | |
|---|---|
| Diagnosis in short | |
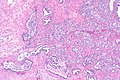
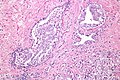
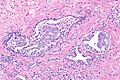
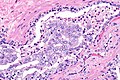
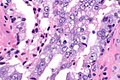
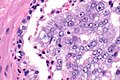
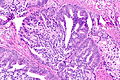
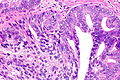
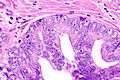
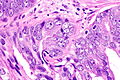
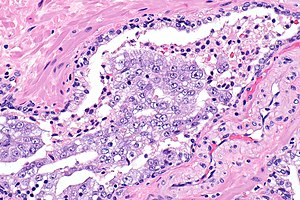 Intraductal carcinoma of prostate. H&E stain. | |
|
| |
| LM | see microscopic section |
| LM DDx | invasive prostatic carcinoma, high grade prostatic intraepithelial neoplasia |
| IHC | basal cells are present (CK34betaE12 +ve, p63 +ve) |
| Molecular | +/-BRCA2 mutation |
| Site | prostate gland |
|
| |
| Prevalence | uncommon |
| Blood work | PSA typically elevated |
| Prognosis | poor, strongly suggestive invasion if not present |
Intraductal carcinoma of the prostate, abbreviated IDC-P, is a proliferation of malignant prostate cells within glands that have an intact basal cell layer.
It should not be confused with ductal adenocarcinoma of the prostate gland.
General
- May represent a precursor to invasive prostate carcinoma.[1]
- Associated with a poor prognosis.[2]
- Strong association with aggressive invasive carcinomas on prostatectomy when identified in isolation on biopsy.[3]
- High interobserver variability among experts ~43% agreement.[4]
- For comparison: HGPIN ~70% agreement, invasive carcinoma ~73% agreement.
- Uncommon finding ~ 3% of cases in a series of 1176 prostate biopsies.[5]
Prevalence
Prevalence varies by risk/stage - based on a systematic review by Porter et al.:[6]
| Group | Prevalence |
|---|---|
| WHO1 † | 2% |
| WHO2 † or WHO3 † | 23% |
| WHO4 † or WHO5 † | 37% |
| Metastasis or biochemical recurrence | 56% |
† WHO1 = WHO grade group 1 (Gleason score 6), WHO2 = WHO grade group 2 or 3 (Gleason score 7 (3+4 or 4+3), WHO3 = WHO grade group 4 or 5 (Gleason score 8, 9 or 10)
Microscopic
Epstein criteria
Epstein's IDCP criteria:[7]
- "Large" acini or ducts with basal cells and one of the following:
- Solid growth pattern or "dense" cribriform pattern.
- Micropapillary pattern or "loose" cribriform pattern with one of the following:
- Marked atypia 6x normal (area).‡
- Non-focal comedonecrosis.
Notes:
- † "Dense" is defined as "more solid than luminal areas"; >50% of the lesion area is cells, as opposed to empty space.
- ‡ Epstein's paper does not explicitly state that it is the area; however, there is near consensus in an ENUP survey that this is the case.[8] The equivalent diameter multiplier is sqrt(6) ~ 2.45; 6x area ~= 2.45x diameter.
Zhou criteria
Major criteria
Required major criteria:[9][10]
- Glands 2x normal (peripheral zone) glands.
- Basal cells present (proven by IHC).
- "Cytologically malignant cells" = nuclear hyperchromasia, nuclear enlargement, nucleoli.
- Fills the lumen ("expansile") but does not have to be "solid".
- Solid = no spaces between the cells.
Additional (major) criterion:[9]
- Comedonecrosis.
Minor criteria
Minor criteria:[9]
- Branching of ducts at right angles.
- Rounded/smooth gland outlines.
- Two cell populations:
- Malignant population (enlarged nuclei with hyperchromasia and nucleoli) = peripheral location in gland.
- Benign population (smaller nuclei, no nucleoli) = central location in gland.
DDx
- High-grade prostatic intraepithelial neoplasia (HGPIN).
- Invasive prostate adenocarcinoma.
Images
Case 1
Case 2
IHC
Features - basal cells present:
- CK34betaE12 +ve.
- p63 +ve.
Molecular
See also
- Intraductal carcinoma.
- Prostate carcinoma.
- Ductal adenocarcinoma of the prostate.
- Cribriform pattern within invasive prostate carcinoma.
References
- ↑ Miyai, K.; Divatia, MK.; Shen, SS.; Miles, BJ.; Ayala, AG.; Ro, JY. (2014). "Heterogeneous clinicopathological features of intraductal carcinoma of the prostate: a comparison between "precursor-like" and "regular type" lesions.". Int J Clin Exp Pathol 7 (5): 2518-26. PMID 24966964.
- ↑ Henry, PC.; Evans, AJ. (Jul 2009). "Intraductal carcinoma of the prostate: a distinct histopathological entity with important prognostic implications.". J Clin Pathol 62 (7): 579-83. doi:10.1136/jcp.2009.065003. PMID 19246509.
- ↑ Robinson, BD.; Epstein, JI. (Oct 2010). "Intraductal carcinoma of the prostate without invasive carcinoma on needle biopsy: emphasis on radical prostatectomy findings.". J Urol 184 (4): 1328-33. doi:10.1016/j.juro.2010.06.017. PMID 20723921.
- ↑ Iczkowski, KA.; Egevad, L.; Ma, J.; Harding-Jackson, N.; Algaba, F.; Billis, A.; Camparo, P.; Cheng, L. et al. (Dec 2014). "Intraductal carcinoma of the prostate: interobserver reproducibility survey of 39 urologic pathologists.". Ann Diagn Pathol 18 (6): 333-42. doi:10.1016/j.anndiagpath.2014.08.010. PMID 25263387.
- ↑ Watts, K.; Li, J.; Magi-Galluzzi, C.; Zhou, M. (Oct 2013). "Incidence and clinicopathological characteristics of intraductal carcinoma detected in prostate biopsies: a prospective cohort study.". Histopathology 63 (4): 574-9. doi:10.1111/his.12198. PMID 23931616.
- ↑ Porter, LH.; Lawrence, MG.; Ilic, D.; Clouston, D.; Bolton, DM.; Frydenberg, M.; Murphy, DG.; Pezaro, C. et al. (Oct 2017). "Systematic Review Links the Prevalence of Intraductal Carcinoma of the Prostate to Prostate Cancer Risk Categories.". Eur Urol 72 (4): 492-495. doi:10.1016/j.eururo.2017.03.013. PMID 28342640.
- ↑ Guo, CC.; Epstein, JI. (Dec 2006). "Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance.". Mod Pathol 19 (12): 1528-35. doi:10.1038/modpathol.3800702. PMID 16980940.
- ↑ Varma M, Egevad L, Berney D, Bubendorf L, Compérat EM, Hes O, van Leenders G, Kristiansen G, Intraductal Carcinoma of the Prostate (IDCP) Reporting Practice: A Survey of Practicing Pathologists. United States and Canadian Academy of Pathology Annual Meeting 2018. Available at: https://www.xcdsystem.com/uscap/program/2018/index.cfm?pgid=1&qfixed=1&SessionType=Platform%20Presentation.
- ↑ 9.0 9.1 9.2 Shah, RB.; Zhou, M. (Jul 2012). "Atypical cribriform lesions of the prostate: clinical significance, differential diagnosis and current concept of intraductal carcinoma of the prostate.". Adv Anat Pathol 19 (4): 270-8. doi:10.1097/PAP.0b013e31825c6c0e. PMID 22692290.
- ↑ Cohen, RJ.; Wheeler, TM.; Bonkhoff, H.; Rubin, MA. (Jul 2007). "A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma.". Arch Pathol Lab Med 131 (7): 1103-9. doi:10.1043/1543-2165(2007)131[1103:APOTIH]2.0.CO;2. PMID 17616999.
- ↑ Taylor RA, Fraser M, Livingstone J, Espiritu SM, Thorne H, Huang V, Lo W, Shiah YJ, Yamaguchi TN, Sliwinski A, Horsburgh S, Meng A, Heisler LE, Yu N, Yousif F, Papargiris M, Lawrence MG, Timms L, Murphy DG, Frydenberg M, Hopkins JF, Bolton D, Clouston D, McPherson JD, van der Kwast T, Boutros PC, Risbridger GP, Bristow RG (January 2017). "Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories". Nat Commun 8: 13671. doi:10.1038/ncomms13671. PMC 5227331. PMID 28067867. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5227331/.