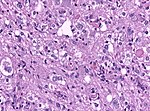
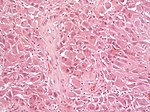
Astrocytoma
Revision as of 13:52, 18 April 2016 by Jensflorian (talk | contribs) (→H3.3 K27M mutated glioma of the midline: + picture)
An astrocytoma is a neoplasm derived from an astrocyte. Astrocytomas are common. This article is a brief introduction them. An overview of CNS tumours is found in the CNS tumours article.
Overview
| Name | Type | Variants / Patterns | Image |
|---|---|---|---|
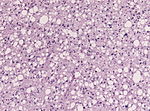
| Diffuse Astrocytoma, WHO II | diffuse | protoplasmatic, fibrillar, gemistocytic | |
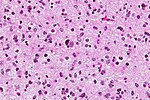
| Anaplastic Astrocytoma, WHO III | diffuse | gliomatosis cerebri | |
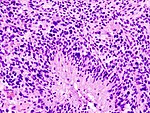
| Glioblastoma, WHO IV | diffuse | small cell, epitheloid/rhabdoid, with PNET componet, with granular cell component, giant cell, gliosarcoma | |
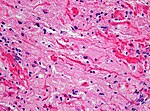
| Pilocytic astrocytoma, WHO I | circumscribed | pilomyxoid astrocytoma, anaplastic pilocytic astrocytoma | |
| Pleomorphic xanthoastrocytoma, WHO II (PXA) | circumscribed | anaplastic PXA | |
| Subependymal giant cell astrocytoma, WHO I (SEGA) | circumscribed | SEGA in tuberous sclerosis |
Common
Pilocytic astrocytoma
- Benign, cystic, infratentorial.
- Classic childhood tumor, surgically resectable.
- Variant: Pilomyxoid astrocytoma
Main article: Pilocytic astrocytoma
Diffuse astrocytoma
- Grade II astrocytic tumors typically seen in adults.
- Usually show progression to glioblastoma.
Main article: Diffuse astrocytoma
Anaplastic astrocytoma
- Grade III astrocytic tumors typically seen in adults.
- Lacks endothelial proliferations and necrosis of glioblastoma.
Main article: Anaplastic astrocytoma
Glioblastoma
- Most common malignant brain tumor peaking around 65 years.
- Prognosis very poor.
- Variant: Giant cell glioblastoma
- Variant: Gliosarcoma
Main article: Glioblastoma
Uncommon
Subependymal giant cell astrocytoma
- Intraventricular benign tumor of adolescents.
- Assoicated with Tuberous sclerosis.
Main article: Subependymal giant cell astrocytoma
Pleomorphic xanthroastrocytoma (PXA)
- Kids & young adults usually with good prognosis.
- Large lipidized cells mimicking a malignant tumor
Main article: Pleomorphic xanthoastrocytoma
Gliomatosis cerebri
- Extensively diffusely growing astrocytic neoplasm.
- Currently considered a rare pattern of diffuse glioma infiltration.
- Introduced in 1938 as a post-mortem diagnosis.[1]
- More than 3 lobes have to be involved, us. bilateral (radiology required).
- biologic behaviour corresponds to WHO III (ICD-O: 9381/3)
- Based on presence / absence of a solid component authors propose two types:[2]
- GC type 1: classic diffuse growth, without IDH1/2 mutation.
- GC type 2: with a solid portion, mostly IDH1 mutant.
- Genetic studies indicate strong overlap with diffuse astrocytic gliomas, oligodendrogliomas and glioblastoma.
- It is likely that suggests that in the upcoming WHO classification gliomatosis is no longer a separate glioma entity.[3]
H3.3 K27M mutated glioma of the midline
- High-grade astrocytic neoplasm associated with midline structures
- Mostly in children and adolescents
- Includes diffuse intrinsic pontine gliomas (DPIG)
- Will become provisonal variant in upcoming WHO 2016 classification
- Distinct biological and clinical group with poor prognosis [4]
Nuclear H3F3A K27M immunostaining in a diffuse glioma of the midline. (WC/jensflorian)
Gliosarcoma
General
- Considered to be a variant of glioblastoma by WHO.[5]
- Rare ~ 200 cases reported in the literature.[5]
- Definition: gliosarcoma = glioblastoma + sarcomatous component.[6]
- Usual location (like glioblastoma): temporal lobe.
Microscopic
Features:
- Glioblastoma.
- Sarcomatous component (one of the following):[5][6]
- Fibroblastic.
- Cartilaginous.
- Osseous.
- Smooth muscle.
- Striated muscle.
- Adipocyte.
Images
www:
- Gliosarcoma - several images (upmc.edu).
- Gliosarcoma - case 2 - several images (upmc.edu).
- Gliosarcoma - case 3 - several images (upmc.edu).
IHC
Gliosarcoma with smooth muscle component (gliomyosarcoma):[9]
- SMA +ve.
- Factor VIII +ve.
Gliofibroma
- Very rare indolent tumor in children [10]
- Usually not dura-based (DD: Desmoplastic infantile astrocytoma)
- Glial tumor with non-neoplastic fibromatous component.
See also
References
- ↑ SAMUEL NEVIN - GLIOMATOSIS CEREBRI, DOI: http://dx.doi.org/10.1093/brain/61.2.170 170-191 First published online: 1 June 1938
- ↑ Seiz, M.; Tuettenberg, J.; Meyer, J.; Essig, M.; Schmieder, K.; Mawrin, C.; von Deimling, A.; Hartmann, C. (Aug 2010). "Detection of IDH1 mutations in gliomatosis cerebri, but only in tumors with additional solid component: evidence for molecular subtypes.". Acta Neuropathol 120 (2): 261-7. doi:10.1007/s00401-010-0701-2. PMID 20514489.
- ↑ Herrlinger, U.; Jones, DT.; Glas, M.; Hattingen, E.; Gramatzki, D.; Stuplich, M.; Felsberg, J.; Bähr, O. et al. (Oct 2015). "Gliomatosis cerebri: no evidence for a separate brain tumor entity.". Acta Neuropathol. doi:10.1007/s00401-015-1495-z. PMID 26493382.
- ↑ Khuong-Quang, DA.; Buczkowicz, P.; Rakopoulos, P.; Liu, XY.; Fontebasso, AM.; Bouffet, E.; Bartels, U.; Albrecht, S. et al. (Sep 2012). "K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas.". Acta Neuropathol 124 (3): 439-47. doi:10.1007/s00401-012-0998-0. PMID 22661320.
- ↑ 5.0 5.1 5.2 Han SJ, Yang I, Tihan T, Prados MD, Parsa AT (February 2010). "Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity". J. Neurooncol. 96 (3): 313–20. doi:10.1007/s11060-009-9973-6. PMC 2808523. PMID 19618114. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2808523/.
- ↑ 6.0 6.1 Ayadi L, Charfi S, Khabir A, et al. (March 2010). "[Cerebral gliosarcoma: clinico-pathologic study of 8 cases]" (in French). Tunis Med 88 (3): 142–6. PMID 20415184.
- ↑ Horiguchi, H.; Hirose, T.; Kannuki, S.; Nagahiro, S.; Sano, T. (Aug 1998). "Gliosarcoma: an immunohistochemical, ultrastructural and fluorescence in situ hybridization study.". Pathol Int 48 (8): 595-602. PMID 9736406.
- ↑ URL: http://path.upmc.edu/cases/case361.html. Accessed on: 15 January 2012.
- ↑ Khanna, M.; Siraj, F.; Chopra, P.; Bhalla, S.; Roy, S.. "Gliosarcoma with prominent smooth muscle component (gliomyosarcoma): a report of 10 cases.". Indian J Pathol Microbiol 54 (1): 51-4. doi:10.4103/0377-4929.77324. PMID 21393877.
- ↑ Deb, P.; Sarkar, C.; Garg, A.; Singh, VP.; Kale, SS.; Sharma, MC. (Feb 2006). "Intracranial gliofibroma mimicking a meningioma: a case report and review of literature.". Clin Neurol Neurosurg 108 (2): 178-86. doi:10.1016/j.clineuro.2004.11.021. PMID 16412839.