Anaplastic astrocytoma
Revision as of 14:33, 19 May 2016 by Jensflorian (talk | contribs) (continued - molecular data is still missing)
Anaplastic astrocytoma (AKA: high-grade astrocytoma) is a infiltrating neoplasm of the diffuse astrocytic and oligodendroglial tumor group occurring in the CNS white matter.
- Most common grade III WHO glioma in adults (peaks between 40-50 years).
- Approx 5% of all gliomas.[1]
- Usually shows progression to glioblastoma sooner or later.
WHO 2016 categorization combines morphology and genetics into following groups:[2]
- Anaplastic astrocytoma, IDH-mutant (ICD-O: 9401/3).
- Anaplastic astrocytoma, IDH-wildtype (ICD-O: 9401/3).
- Anaplastic astrocytoma,NOS (ICD-O: 9401/3) - genetic data missing.
Radiology/Clinic
- Mass effect.
- Seizures.
- Neurologic decifit.
- The majority are contrast-enhanching, T2 bright.
Prognosis
- Overall prognosis is rather poor (average survival 2-3 years).
- IDH-mutant tumors share a similiar prognosis to grade II IDH-mutant tumors.[3]
- Anaplastic astrocytoma, IDH-wildtype perform worse than glioblastoma, IDH-mutant despite grading differences.[4]
Macroscopy
- No clear demarcation from white matter.
- Invaded structures may appear enlarged.
- Softer consistency and opacity.
- No necrosis.
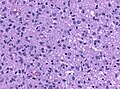
Histology
Features: [5]
- Increased cellularity (compared to Diffuse Astrocytoma).
- Specimens with low cellularity but plenty mitoses are also considered anaplastic.
- Distinct nuclear atypia and pleomorphism.
- May include multinucleated cells.
- Cytoplasm highly variable (even within the same tumour).
- Mitoses present (a single mitosis in a small specimen indicates a high-grade tumor).
- Microcystic spaces of the background (none to extensive).
- No necrosis, no vascular proliferations.
- Except radiation necrosis after pretreatment.
- Lymphocytic cuffing (mostly in gemistocytic type).
- Rosenthal fibers usu. absent.
IHC
- GFAP+ve.
- MAP2+ve (especially in cell processes).
- Vimentin+ve (often perinuclear).
- S-100+ve.
- MIB-1: usu. 5-10& (overlaps with grade II tumors).
- IDH-1 (R132H)+ve in 60-70%.
- 'Note: This antibody does not detect other rare IDH1/2 mutations.
- ATRX nuclear loss in 70%.
DDx
- Diffuse astrocytoma - absent or very low mitotic activity.
- Anaplastic Oligoastrocytoma, NOS - esp. when genetic data on IDH and LOH 1p/19q are lacking.
- Anaplastic Oligodendroglioma, when LOH 1p/19q is present.
- Glioblastoma - vascular proliferations and / or necrosis.
- ↑ Ohgaki, H.; Kleihues, P. (Jun 2005). "Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas.". J Neuropathol Exp Neurol 64 (6): 479-89. PMID 15977639.
- ↑ Louis, DN.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, WK.; Ohgaki, H.; Wiestler, OD. et al. (Jun 2016). "The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary.". Acta Neuropathol 131 (6): 803-20. doi:10.1007/s00401-016-1545-1. PMID 27157931.
- ↑ Reuss, DE.; Mamatjan, Y.; Schrimpf, D.; Capper, D.; Hovestadt, V.; Kratz, A.; Sahm, F.; Koelsche, C. et al. (Jun 2015). "IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO.". Acta Neuropathol 129 (6): 867-73. doi:10.1007/s00401-015-1438-8. PMID 25962792.
- ↑ Hartmann, C.; Hentschel, B.; Wick, W.; Capper, D.; Felsberg, J.; Simon, M.; Westphal, M.; Schackert, G. et al. (Dec 2010). "Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas.". Acta Neuropathol 120 (6): 707-18. doi:10.1007/s00401-010-0781-z. PMID 21088844.
- ↑ Burger, P.C.; Scheithauer, B.W. (2007). Tumors of the Central Nervous System (Afip Atlas of Tumor Pathology) (4th ed.). Washington: American Registry of Pathology. pp. 34. ISBN 1933477016.