Difference between revisions of "Astrocytoma"
Jump to navigation
Jump to search
Jensflorian (talk | contribs) (update) |
Jensflorian (talk | contribs) (→Overview: Missing image added) |
||
| Line 42: | Line 42: | ||
| circumscribed | | circumscribed | ||
| | | | ||
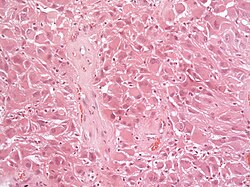
| | | [[File:Anaplastic_pxa_histology.jpg|thumb|center|150px]] | ||
|- | |- | ||
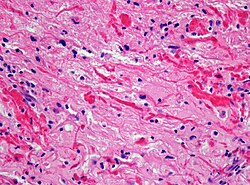
| Subependymal giant cell astrocytoma, WHO I (SEGA) | | Subependymal giant cell astrocytoma, WHO I (SEGA) | ||
| Line 49: | Line 49: | ||
| [[File:SEGA_HE.jpg|thumb|center|150px]] | | [[File:SEGA_HE.jpg|thumb|center|150px]] | ||
|} | |} | ||
=Common= | =Common= | ||
==Pilocytic astrocytoma== | ==Pilocytic astrocytoma== | ||
Revision as of 12:35, 11 October 2019
An astrocytoma is a neoplasm derived from an astrocyte. Diffuse astrocytomas are common glial tumours and grouped together with Oligodendroglioma in the current WHO brain tumor classficiation. Some (often circumscribed) astrocytic tumors are biologically different from diffuse astrocytomas An overview of CNS tumours is found in the CNS tumours article.
Overview
| Name | Type | Variants / Patterns | Image |
|---|---|---|---|
| Diffuse Astrocytoma, WHO II | diffuse | protoplasmatic, fibrillar, gemistocytic | |
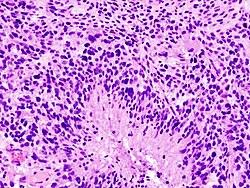
| Anaplastic Astrocytoma, WHO III | diffuse | gliomatosis cerebri | |
| Glioblastoma, WHO IV | diffuse | small cell, epitheloid/rhabdoid, with PNET componet, with granular cell component, giant cell, gliosarcoma | |
| Diffuse midline glioma, H3 K27M-mutant, WHO IV | diffuse | ||
| Pilocytic astrocytoma, WHO I | circumscribed | pilomyxoid astrocytoma, anaplastic pilocytic astrocytoma | |
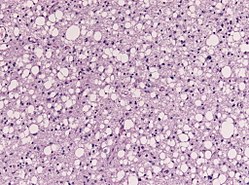
| Pleomorphic xanthoastrocytoma, WHO II (PXA) | circumscribed | ||
| Anaplastic pleomorphic xanthoastrocytoma, WHO III (PXA) | circumscribed | ||
| Subependymal giant cell astrocytoma, WHO I (SEGA) | circumscribed | SEGA in tuberous sclerosis |
Common
Pilocytic astrocytoma
- Benign, cystic, infratentorial.
- Classic childhood tumor, surgically resectable.
- Variant: Pilomyxoid astrocytoma
Main article: Pilocytic astrocytoma
Diffuse astrocytoma
- Grade II astrocytic tumors typically seen in adults.
- Usually show progression to glioblastoma.
Main article: Diffuse astrocytoma
Anaplastic astrocytoma
- Grade III astrocytic tumors typically seen in adults.
- Lacks endothelial proliferations and necrosis of glioblastoma.
Main article: Anaplastic astrocytoma
Glioblastoma
- Most common malignant brain tumor peaking around 65 years.
- Prognosis very poor.
- Variant: Giant cell glioblastoma
- Variant: Gliosarcoma
Main article: Glioblastoma
Uncommon
Subependymal giant cell astrocytoma
- Intraventricular benign tumor of adolescents.
- Assoicated with Tuberous sclerosis.
Main article: Subependymal giant cell astrocytoma
Pleomorphic xanthroastrocytoma (PXA)
- Kids & young adults usually with good prognosis.
- Large lipidized cells mimicking a malignant tumor
Main article: Pleomorphic xanthoastrocytoma
Gliomatosis cerebri
- Depreceated entity.
- Was used for extensively diffusely growing astrocytic neoplasms.
- More than 3 lobes have to be involved, us. bilateral (radiology required).
- biologic behaviour corresponds to WHO III (ICD-O: 9381/3)
- Based on presence / absence of a solid component authors propose two types:[4]
- GC type 1: classic diffuse growth, without IDH1/2 mutation.
- GC type 2: with a solid portion, mostly IDH1 mutant.
- Genetic studies indicate strong overlap with diffuse astrocytic gliomas, oligodendrogliomas and glioblastoma.
Diffuse midline glioma, H3 K27M mutant
- High-grade astrocytic neoplasm associated with midline structures (thalamus, brain stem, spinal cord).
- Mostly in children and adolescents.
- Includes diffuse intrinsic pontine gliomas (DPIG).
- Newly defined entity since WHO 2016 classification.[5]
- Distinct biological and clinical group with poor prognosis.[6]
- MRI: May be or be not enhancing.
- Histologic spectrum ranges from minimal hypercellularity to full-blown glioblastoma.
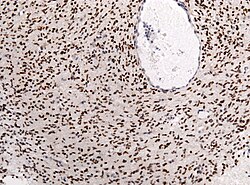
Note: Cases may also appear outside midline structures and in adult patients.[9]
Nuclear H3F3A K27M immunostaining in a diffuse glioma of the midline. (WC/jensflorian)
Gliosarcoma
General
- Considered to be a variant of glioblastoma by WHO.[10]
- Rare ~ 200 cases reported in the literature.[10]
- Definition: gliosarcoma = glioblastoma + sarcomatous component.[11]
- Usual location (like glioblastoma): temporal lobe.
- Prognosis is similiar to glioblastoma.[12]
- Age below 65 years is prognostic.
Microscopic
Features:
- Glioblastoma.
- Sarcomatous component (one of the following):[10][11]
- Fibroblastic.
- Cartilaginous.
- Osseous.
- Smooth muscle.
- Striated muscle.
- Adipocyte.
Images
www:
- Gliosarcoma - several images (upmc.edu).
- Gliosarcoma - case 2 - several images (upmc.edu).
- Gliosarcoma - case 3 - several images (upmc.edu).
IHC
Gliosarcoma with smooth muscle component (gliomyosarcoma):[15]
- SMA +ve.
- Factor VIII +ve.
Gliofibroma
- Very rare indolent tumor in children [16]
- Usually not dura-based (DD: Desmoplastic infantile astrocytoma)
- Glial tumor with non-neoplastic fibromatous component.
See also
References
- ↑ SAMUEL NEVIN - GLIOMATOSIS CEREBRI, DOI: http://dx.doi.org/10.1093/brain/61.2.170 170-191 First published online: 1 June 1938
- ↑ Johnson, DR.; Guerin, JB.; Giannini, C.; Morris, JM.; Eckel, LJ.; Kaufmann, TJ.. "2016 Updates to the WHO Brain Tumor Classification System: What the Radiologist Needs to Know.". Radiographics 37 (7): 2164-2180. doi:10.1148/rg.2017170037. PMID 29028423.
- ↑ Herrlinger, U.; Jones, DT.; Glas, M.; Hattingen, E.; Gramatzki, D.; Stuplich, M.; Felsberg, J.; Bähr, O. et al. (Oct 2015). "Gliomatosis cerebri: no evidence for a separate brain tumor entity.". Acta Neuropathol. doi:10.1007/s00401-015-1495-z. PMID 26493382.
- ↑ Seiz, M.; Tuettenberg, J.; Meyer, J.; Essig, M.; Schmieder, K.; Mawrin, C.; von Deimling, A.; Hartmann, C. (Aug 2010). "Detection of IDH1 mutations in gliomatosis cerebri, but only in tumors with additional solid component: evidence for molecular subtypes.". Acta Neuropathol 120 (2): 261-7. doi:10.1007/s00401-010-0701-2. PMID 20514489.
- ↑ Louis, DN.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, WK.; Ohgaki, H.; Wiestler, OD. et al. (Jun 2016). "The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary.". Acta Neuropathol 131 (6): 803-20. doi:10.1007/s00401-016-1545-1. PMID 27157931.
- ↑ Khuong-Quang, DA.; Buczkowicz, P.; Rakopoulos, P.; Liu, XY.; Fontebasso, AM.; Bouffet, E.; Bartels, U.; Albrecht, S. et al. (Sep 2012). "K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas.". Acta Neuropathol 124 (3): 439-47. doi:10.1007/s00401-012-0998-0. PMID 22661320.
- ↑ Meyronet, D.; Esteban-Mader, M.; Bonnet, C.; Joly, MO.; Uro-Coste, E.; Amiel-Benouaich, A.; Forest, F.; Rousselot-Denis, C. et al. (Aug 2017). "Characteristics of H3 K27M-mutant gliomas in adults.". Neuro Oncol 19 (8): 1127-1134. doi:10.1093/neuonc/now274. PMID 28201752.
- ↑ Banan, R.; Christians, A.; Bartels, S.; Lehmann, U.; Hartmann, C. (12 2017). "Absence of MGMT promoter methylation in diffuse midline glioma, H3 K27M-mutant.". Acta Neuropathol Commun 5 (1): 98. doi:10.1186/s40478-017-0500-2. PMID 29246238.
- ↑ Nakata, S.; Nobusawa, S.; Yamazaki, T.; Osawa, T.; Horiguchi, K.; Hashiba, Y.; Yaoita, H.; Matsumura, N. et al. (Jul 2017). "Histone H3 K27M mutations in adult cerebellar high-grade gliomas.". Brain Tumor Pathol 34 (3): 113-119. doi:10.1007/s10014-017-0288-6. PMID 28547652.
- ↑ Jump up to: 10.0 10.1 10.2 Han SJ, Yang I, Tihan T, Prados MD, Parsa AT (February 2010). "Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity". J. Neurooncol. 96 (3): 313–20. doi:10.1007/s11060-009-9973-6. PMC 2808523. PMID 19618114. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2808523/.
- ↑ Jump up to: 11.0 11.1 Ayadi L, Charfi S, Khabir A, et al. (March 2010). "[Cerebral gliosarcoma: clinico-pathologic study of 8 cases]" (in French). Tunis Med 88 (3): 142–6. PMID 20415184.
- ↑ Frandsen, J.; Orton, A.; Jensen, R.; Colman, H.; Cohen, AL.; Tward, J.; Shrieve, DC.; Suneja, G. (Jun 2017). "Patterns of care and outcomes in gliosarcoma: an analysis of the National Cancer Database.". J Neurosurg: 1-6. doi:10.3171/2016.12.JNS162291. PMID 28621623.
- ↑ Horiguchi, H.; Hirose, T.; Kannuki, S.; Nagahiro, S.; Sano, T. (Aug 1998). "Gliosarcoma: an immunohistochemical, ultrastructural and fluorescence in situ hybridization study.". Pathol Int 48 (8): 595-602. PMID 9736406.
- ↑ URL: http://path.upmc.edu/cases/case361.html. Accessed on: 15 January 2012.
- ↑ Khanna, M.; Siraj, F.; Chopra, P.; Bhalla, S.; Roy, S.. "Gliosarcoma with prominent smooth muscle component (gliomyosarcoma): a report of 10 cases.". Indian J Pathol Microbiol 54 (1): 51-4. doi:10.4103/0377-4929.77324. PMID 21393877.
- ↑ Deb, P.; Sarkar, C.; Garg, A.; Singh, VP.; Kale, SS.; Sharma, MC. (Feb 2006). "Intracranial gliofibroma mimicking a meningioma: a case report and review of literature.". Clin Neurol Neurosurg 108 (2): 178-86. doi:10.1016/j.clineuro.2004.11.021. PMID 16412839.