Difference between revisions of "Colorectal tumours"
Jump to navigation
Jump to search
(→Others: mucinous carcinoma note) |
|||
| (112 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Colorectal tumours''' are very common. They are the bread and butter of GI pathology. Non-tumour colon is dealt with in the ''[[colon]]'' article. | '''Colorectal tumours''', especially '''colorectal carcinomas''', are very common. They are the bread and butter of GI pathology. Non-tumour colon is dealt with in the ''[[colon]]'' article. | ||
''Colonic tumours'' and ''rectal tumours'' redirect here. | |||
An introduction to gastrointestinal pathology is in the ''[[gastrointestinal pathology]]'' article. The precursor lesion of colorectal carcinoma (CRC) is, typically, an [[adenomatous polyps|adenomatous polyp]]. Polyps are discussed in the ''[[intestinal polyps]]'' article. | |||
=Classification= | |||
===Most common=== | ===Most common=== | ||
*Colon & rectum - most common = [[adenocarcinoma]].<ref>{{Ref PBoD|864}}</ref> | *Colon & rectum - most common = [[colorectal adenocarcinoma|adenocarinoma]].<ref name=Ref_PBoD864>{{Ref PBoD|864}}</ref> | ||
===Others=== | ===Others=== | ||
Other tumours - many (incomplete list):<ref>{{Ref WMSP|198}}</ref> | Other tumours - many (incomplete list):<ref name=Ref_WMSP198>{{Ref WMSP|198}}</ref> | ||
*Mucinous carcinoma. | *[[Mucinous carcinoma]]. | ||
**Need > 50% mucinous component.<ref name=pmid17679024 >{{cite journal |author=Tozawa E, Ajioka Y, Watanabe H, ''et al.'' |title=Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: is mucinous carcinoma a distinct histological entity? |journal=Pathol. Res. Pract. |volume=203 |issue=8 |pages=567–74 |year=2007 |pmid=17679024 |doi=10.1016/j.prp.2007.04.013 |url=}}</ref> | **Need > 50% mucinous component.<ref name=pmid17679024 >{{cite journal |author=Tozawa E, Ajioka Y, Watanabe H, ''et al.'' |title=Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: is mucinous carcinoma a distinct histological entity? |journal=Pathol. Res. Pract. |volume=203 |issue=8 |pages=567–74 |year=2007 |pmid=17679024 |doi=10.1016/j.prp.2007.04.013 |url=}}</ref> | ||
*Adenosquamous carcinoma. | *[[Adenosquamous carcinoma]]. | ||
*Signet-ring carcinoma. | *Signet-ring carcinoma. | ||
*Squamous carcinoma. | *Squamous carcinoma. | ||
*Neuroendocrine | *[[Neuroendocrine neoplasm]]s (carcinoid tumours). | ||
*[[Lipoma]]. | *[[Lipoma]]. | ||
*[[Leiomyoma]]. | *[[Leiomyoma]]. | ||
| Line 22: | Line 24: | ||
Notes: | Notes: | ||
*Mucinous carcinoma - percentage required to call varies by site: | *[[Mucinous carcinoma]] - percentage required to call varies by site: | ||
====Squamous carcinoma==== | ====Squamous carcinoma==== | ||
{{Main|Squamous carcinoma}} | |||
*Rare. | *Rare. | ||
**In the context of a rectal tumour, retrograde growth from the [[anus]] should be considered. | **In the context of a rectal tumour, retrograde growth from the [[anus]] should be considered. | ||
==Staging of colorectal cancer== | ==Staging of colorectal cancer== | ||
{{Main|Colorectal cancer staging}} | |||
==Pathogenesis== | ==Pathogenesis of colorectal carcinoma== | ||
===Overview=== | ===Overview=== | ||
Colorectal carcinoma is thought to arise from one of two pathways:<ref name=pmid16483003>{{cite journal |author=Goldstein NS |title=Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification |journal=Am. J. Clin. Pathol. |volume=125 |issue=1 |pages=146–53 |year=2006 |month=January |pmid=16483003 |doi= |url=}}</ref><ref name=pmid18314605>{{cite journal |author=Rüschoff J, Aust D, Hartmann A |title=[Colorectal serrated adenoma: diagnostic criteria and clinical implications] |language=German |journal=Verh Dtsch Ges Pathol |volume=91 |issue= |pages=119–25 |year=2007 |pmid=18314605 |doi= |url=}}</ref> | Colorectal carcinoma is thought to arise from one of two pathways:<ref name=pmid16483003>{{cite journal |author=Goldstein NS |title=Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification |journal=Am. J. Clin. Pathol. |volume=125 |issue=1 |pages=146–53 |year=2006 |month=January |pmid=16483003 |doi= |url=}}</ref><ref name=pmid18314605>{{cite journal |author=Rüschoff J, Aust D, Hartmann A |title=[Colorectal serrated adenoma: diagnostic criteria and clinical implications] |language=German |journal=Verh Dtsch Ges Pathol |volume=91 |issue= |pages=119–25 |year=2007 |pmid=18314605 |doi= |url=}}</ref> | ||
| Line 73: | Line 40: | ||
#Serrated pathway, AKA mutator pathway, mismatch repair pathway. | #Serrated pathway, AKA mutator pathway, mismatch repair pathway. | ||
=== | ====Syndromes==== | ||
Both of the above described pathways are associated with syndromes: | |||
#''[[Familial adenomatous polyposis]]'' (FAP) or ''familial polyposis coli'' (FPC). | |||
# | #''Lynch syndrome'' ([[AKA]] ''[[hereditary non-polyposis colorectal cancer syndrome]]'' (HNPCC)). | ||
# | |||
===Pathways=== | |||
====APC gene mutation pathway==== | |||
Microscopic: | |||
*[[Adenomatous polyps]]. | |||
====Mismatch repair pathway==== | |||
* | *Associated with [[microsatellite instability]] (MSI). | ||
===Other ancillary studies=== | ===Other ancillary studies=== | ||
*BRAF ''V600E'' missense mutation found in ~10% CRC.<ref name=pmid20635392>{{cite journal |author=Tie J, Gibbs P, Lipton L, ''et al.'' |title=Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation |journal=Int J Cancer |volume= |issue= |pages= |year=2010 |month=July |pmid=20635392 |doi=10.1002/ijc.25555 |url=}}</ref> | *BRAF ''V600E'' missense mutation found in ~10% CRC.<ref name=pmid20635392>{{cite journal |author=Tie J, Gibbs P, Lipton L, ''et al.'' |title=Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation |journal=Int J Cancer |volume= |issue= |pages= |year=2010 |month=July |pmid=20635392 |doi=10.1002/ijc.25555 |url=}}</ref> | ||
*KRAS mutation status. | *[[KRAS mutation]] status. | ||
====BRAF V600E mutation==== | ====BRAF V600E mutation==== | ||
{{Main|BRAF V600E mutation}} | |||
Features:<ref name=pmid20635392/> | Features:<ref name=pmid20635392/> | ||
*Independently | *Independently associated with BRAF V600E: | ||
**Usually older (>70 years old). | **Usually older (>70 years old). | ||
**Female gender | **Female gender. | ||
**Right-sided tumour location. | **Right-sided tumour location. | ||
*Worse prognosis - in the context of metastatic disease. | *Worse prognosis - in the context of metastatic disease. | ||
====KRAS mutation==== | ====KRAS mutation==== | ||
{{Main|KRAS mutation}} | |||
Features:<ref name=pmid20956938>{{cite journal |author=Dunn EF, Iida M, Myers RA, ''et al.'' |title=Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab |journal=Oncogene |volume= |issue= |pages= |year=2010 |month=October |pmid=20956938 |doi=10.1038/onc.2010.430 |url=}}</ref><ref name=pmid19001320>{{cite journal |author=Di Nicolantonio F, Martini M, Molinari F, ''et al.'' |title=Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer |journal=J. Clin. Oncol. |volume=26 |issue=35 |pages=5705–12 |year=2008 |month=December |pmid=19001320 |doi=10.1200/JCO.2008.18.0786 |url=}}</ref> | Features:<ref name=pmid20956938>{{cite journal |author=Dunn EF, Iida M, Myers RA, ''et al.'' |title=Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab |journal=Oncogene |volume= |issue= |pages= |year=2010 |month=October |pmid=20956938 |doi=10.1038/onc.2010.430 |url=}}</ref><ref name=pmid19001320>{{cite journal |author=Di Nicolantonio F, Martini M, Molinari F, ''et al.'' |title=Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer |journal=J. Clin. Oncol. |volume=26 |issue=35 |pages=5705–12 |year=2008 |month=December |pmid=19001320 |doi=10.1200/JCO.2008.18.0786 |url=}}</ref> | ||
*Patient must have ''wild type'' KRAS to get drugs; KRAS mutation predicts resistance to cetuximab (Erbitux) and panitumumab (Vectibix). | *Patient must have ''wild type'' KRAS to get drugs; KRAS mutation predicts resistance to [[cetuximab]] (Erbitux) and [[panitumumab]] (Vectibix). | ||
**Cetuximab and panitumumab are EGFR inhibitors. | **Cetuximab and panitumumab are [[EGFR inhibitors]]. | ||
== | ==Microsatellite instability cancers== | ||
*Abbreviated ''MSI cancers''. | |||
{{Main|Microsatellite instability in colorectal cancer}} | |||
==== | =Specific entities= | ||
==Colorectal adenocarcinoma== | |||
*[[AKA]] ''colorectal adenocarcinoma not otherwise specified''. | |||
*[[AKA]] ''colorectal carcinoma'', abbreviated ''CRC''. | |||
{{Main|Colorectal adenocarcinoma}} | |||
== | ==Secondary colorectal cancer== | ||
===General=== | |||
* | *Uncommon. | ||
*May be suspected. | |||
===Microscopic=== | ===Microscopic=== | ||
Features: | Features: | ||
* | *Normal colorectal mucosa. | ||
*Atypical cells in the lamina propria or submucosa. | |||
* | DDx: | ||
*Colorectal neuroendocrine tumour. | |||
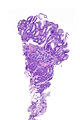
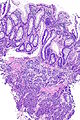
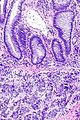
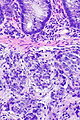
=== | ===Images=== | ||
<gallery> | |||
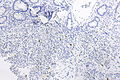
Image:Prostate carcinoma in rectum -- very low mag.jpg | Pca in rectum - very low mag. (WC) | |||
Image:Prostate carcinoma in rectum -- low mag.jpg | Pca in rectum - low mag. (WC) | |||
Image:Prostate carcinoma in rectum -- intermed mag.jpg | Pca in rectum - intermed. mag. (WC) | |||
Image:Prostate carcinoma in rectum -- high mag.jpg | Pca in rectum - high mag. (WC) | |||
</gallery> | |||
<gallery> | |||
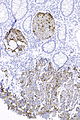
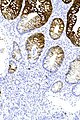
Image:Prostate carcinoma in rectum - PSAP -- intermed mag.jpg | Pca in rectum - PSAP - intermed. mag. (WC) | |||
Image:Prostate carcinoma in rectum - PSA -- intermed mag.jpg | Pca in rectum - PSA - intermed. mag. (WC) | |||
Image:Prostate carcinoma in rectum - CK20 -- intermed mag.jpg | Pca in rectum - CK20 - intermed. mag. (WC) | |||
</gallery> | |||
=See also= | |||
*[[Anus]] - covers anal cancer and anal intraepithelial neoplasia. | *[[Anus]] - covers anal cancer and anal intraepithelial neoplasia. | ||
*[[Colon]]. | *[[Colon]]. | ||
*[[Gastrointestinal pathology]]. | *[[Gastrointestinal pathology]]. | ||
*[[Tumour budding]]. | |||
*[[Tumour perforation in colorectal cancer]]. | |||
*[[Transanal minimally invasive surgery]]. | |||
=References= | |||
{{reflist|2}} | {{reflist|2}} | ||
[[Category:Gastrointestinal pathology]] | [[Category:Gastrointestinal pathology]] | ||
Latest revision as of 16:50, 29 August 2018
Colorectal tumours, especially colorectal carcinomas, are very common. They are the bread and butter of GI pathology. Non-tumour colon is dealt with in the colon article.
Colonic tumours and rectal tumours redirect here.
An introduction to gastrointestinal pathology is in the gastrointestinal pathology article. The precursor lesion of colorectal carcinoma (CRC) is, typically, an adenomatous polyp. Polyps are discussed in the intestinal polyps article.
Classification
Most common
- Colon & rectum - most common = adenocarinoma.[1]
Others
Other tumours - many (incomplete list):[2]
- Mucinous carcinoma.
- Need > 50% mucinous component.[3]
- Adenosquamous carcinoma.
- Signet-ring carcinoma.
- Squamous carcinoma.
- Neuroendocrine neoplasms (carcinoid tumours).
- Lipoma.
- Leiomyoma.
- Gastrointestinal stromal tumour (GIST) - dealt with in a separate article.
- Angiosarcoma.
- Lymphoma (Non-Hodgkin's lymphoma).
Notes:
- Mucinous carcinoma - percentage required to call varies by site:
Squamous carcinoma
Main article: Squamous carcinoma
- Rare.
- In the context of a rectal tumour, retrograde growth from the anus should be considered.
Staging of colorectal cancer
Main article: Colorectal cancer staging
Pathogenesis of colorectal carcinoma
Overview
Colorectal carcinoma is thought to arise from one of two pathways:[4][5]
- APC (adenomatous polyposis coli) gene mutation pathway, AKA classic adenoma-carcinoma pathway.
- Serrated pathway, AKA mutator pathway, mismatch repair pathway.
Syndromes
Both of the above described pathways are associated with syndromes:
- Familial adenomatous polyposis (FAP) or familial polyposis coli (FPC).
- Lynch syndrome (AKA hereditary non-polyposis colorectal cancer syndrome (HNPCC)).
Pathways
APC gene mutation pathway
Microscopic:
Mismatch repair pathway
- Associated with microsatellite instability (MSI).
Other ancillary studies
- BRAF V600E missense mutation found in ~10% CRC.[6]
- KRAS mutation status.
BRAF V600E mutation
Main article: BRAF V600E mutation
Features:[6]
- Independently associated with BRAF V600E:
- Usually older (>70 years old).
- Female gender.
- Right-sided tumour location.
- Worse prognosis - in the context of metastatic disease.
KRAS mutation
Main article: KRAS mutation
- Patient must have wild type KRAS to get drugs; KRAS mutation predicts resistance to cetuximab (Erbitux) and panitumumab (Vectibix).
- Cetuximab and panitumumab are EGFR inhibitors.
Microsatellite instability cancers
- Abbreviated MSI cancers.
Main article: Microsatellite instability in colorectal cancer
Specific entities
Colorectal adenocarcinoma
Main article: Colorectal adenocarcinoma
Secondary colorectal cancer
General
- Uncommon.
- May be suspected.
Microscopic
Features:
- Normal colorectal mucosa.
- Atypical cells in the lamina propria or submucosa.
DDx:
- Colorectal neuroendocrine tumour.
Images
See also
- Anus - covers anal cancer and anal intraepithelial neoplasia.
- Colon.
- Gastrointestinal pathology.
- Tumour budding.
- Tumour perforation in colorectal cancer.
- Transanal minimally invasive surgery.
References
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 864. ISBN 0-7216-0187-1.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 198. ISBN 978-0781765275.
- ↑ Tozawa E, Ajioka Y, Watanabe H, et al. (2007). "Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: is mucinous carcinoma a distinct histological entity?". Pathol. Res. Pract. 203 (8): 567–74. doi:10.1016/j.prp.2007.04.013. PMID 17679024.
- ↑ Goldstein NS (January 2006). "Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification". Am. J. Clin. Pathol. 125 (1): 146–53. PMID 16483003.
- ↑ Rüschoff J, Aust D, Hartmann A (2007). "[Colorectal serrated adenoma: diagnostic criteria and clinical implications]" (in German). Verh Dtsch Ges Pathol 91: 119–25. PMID 18314605.
- ↑ 6.0 6.1 Tie J, Gibbs P, Lipton L, et al. (July 2010). "Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation". Int J Cancer. doi:10.1002/ijc.25555. PMID 20635392.
- ↑ Dunn EF, Iida M, Myers RA, et al. (October 2010). "Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab". Oncogene. doi:10.1038/onc.2010.430. PMID 20956938.
- ↑ Di Nicolantonio F, Martini M, Molinari F, et al. (December 2008). "Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer". J. Clin. Oncol. 26 (35): 5705–12. doi:10.1200/JCO.2008.18.0786. PMID 19001320.