Difference between revisions of "Sertoli-Leydig cell tumour"
(→IHC) |
|||
| Line 18: | Line 18: | ||
#** Irregular nuclei with irregular/vacuolated-appearing chromatin. | #** Irregular nuclei with irregular/vacuolated-appearing chromatin. | ||
#** Architecture: tubules, cords or sheets. | #** Architecture: tubules, cords or sheets. | ||
#**Mitotic activity may be much lower than expected for the degree of atypia (in comparison to adenocarcinoma). | |||
# Stroma. | # Stroma. | ||
#*Stromal edema may be prominent | |||
# +/- Sarcomatous features (mucinous glands, bone, cartilage). | # +/- Sarcomatous features (mucinous glands, bone, cartilage). | ||
Growth Patterns: | Growth Patterns: | ||
Revision as of 11:15, 21 March 2015
Sertoli-Leydig cell tumour, also Sertoli-Leydig tumour, is a rare tumour of the gonad in the sex cord-stromal group of tumours.
General
- Sertoli and leydig cells are normal in the testis.
- Poorly differentiated tumours have sarcomatous features.[1]
- May present with masculinization (virilization).[2]
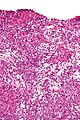
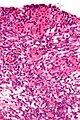
Microscopic
Features:
- Sertoli or Leydig cells.[1]
- Leydig cells:
- Polygonal pink cells
- Abundant solid or somewhat granular eosinophilic cytoplasm.
- Round nuclei with fine chromatin and a small or indistinct nucleolus.
- Often in small clusters ~ 5-25 cells/cluster.
- Sertoli cells:
- Pale/clear vacuolated cytoplasm.
- Irregular nuclei with irregular/vacuolated-appearing chromatin.
- Architecture: tubules, cords or sheets.
- Mitotic activity may be much lower than expected for the degree of atypia (in comparison to adenocarcinoma).
- Leydig cells:
- Stroma.
- Stromal edema may be prominent
- +/- Sarcomatous features (mucinous glands, bone, cartilage).
Growth Patterns:
- Well-differentiated
- Hollow or solid tubules of mature Sertoli cells with Leydig cells in the intervening stroma
- Intermediate (most common)
- Jumbled admixture of dark blue Sertoli cells and Leydig cells
- Lobules comprising sheets of Sertoli cells
- Some areas of tubules.
- Poorly differentiated
- Masses of malignant spindle cells – sheets of cells can be reminiscent of fibrosarcoma or granulosa cell tumour
- Tubules may be a very minor element
- With heterologous element
- Mucinous intestinal-type epithelium, cartilage, skeletal muscle
- Heterologous elements can also occur with retiform or poorly differentiated tumours
- Retiform
- Tumour resembles rete testis/ovary with blunt papillae and irregular cleft-like spaces
- Well-differentiated
DDx:
- Endometrioid carcinoma of the ovary (sertoliform variant) - should be positive for EMA, negative for inhibin and calretinin.
- Luteinized adult granulosa cell tumour - super rare, 50% of cell with eosinophilic cytoplasm, other findings of granulosa cell tumour, e.g. Call-Exner bodies. More likely to be keratin negative than a Sertoli-Leydig cell tumor. [3]
- Ovarian carcinosarcoma - especially considering poorly differentiated versions with heterologous areas.
Images
www:
IHC
Features:[4]
- Inhibin +ve
- Calretinin +ve.
- WT-1 +ve.
- Melan A (MART-1) +ve - marks the Leydig component.
- Vimentin +ve.[5]
- CD99 +ve.
- AE1/AE3 and PanKeratin +ve
Others:[5]
- CD34 -ve.
- EMA -ve.
Keep in mind that this is a biphasic tumor - Leydig cells will not be Pan-keratin positive - Sertoli cells do not express calretinin - Both components express inhibin - etcetera - interpreting this immunopanal requires correlation with the histomorphology.
Pan-keratins and AE1/AE3 may mark granulosa cell tumors and Sertoli cell tumors causing confusion with adenocarcinoma. EMA is a better marker to exclude an epithelial tumor as EMA is negative in sex cord-stromal tumors. Highlighting why a panel of stains is needed, endometrioid adenocarcinomas may occasionally weakly express inhibin, calretinin or WT-1.
See also
References
- ↑ 1.0 1.1 Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 1103. ISBN 0-7216-0187-1.
- ↑ Xiao, H.; Li, B.; Zuo, J.; Feng, X.; Li, X.; Zhang, R.; Wu, L. (Mar 2013). "Ovarian Sertoli-Leydig cell tumor: a report of seven cases and a review of the literature.". Gynecol Endocrinol 29 (3): 192-5. doi:10.3109/09513590.2012.738723. PMID 23173550.
- ↑ Ganesan, R.; Hirschowitz, L.; Baltrušaitytė, I.; McCluggage, WG. (Sep 2011). "Luteinized adult granulosa cell tumor--a series of 9 cases: revisiting a rare variant of adult granulosa cell tumor.". Int J Gynecol Pathol 30 (5): 452-9. doi:10.1097/PGP.0b013e318214b17f. PMID 21804396.
- ↑ Zhao, C.; Vinh, TN.; McManus, K.; Dabbs, D.; Barner, R.; Vang, R. (Mar 2009). "Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors.". Am J Surg Pathol 33 (3): 354-66. doi:10.1097/PAS.0b013e318188373d. PMID 19033865.
- ↑ 5.0 5.1 Kondi-Pafiti, A.; Grapsa, D.; Kairi-Vassilatou, E.; Carvounis, E.; Hasiakos, D.; Kontogianni, K.; Fotiou, S. (2010). "Granulosa cell tumors of the ovary: a clinicopathologic and immunohistochemical study of 21 cases.". Eur J Gynaecol Oncol 31 (1): 94-8. PMID 20349790.