Rhabdomyosarcoma
| Rhabdomyosarcoma | |
|---|---|
| Diagnosis in short | |
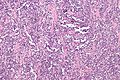
 Alveolar rhabdomyosarcoma. H&E stain. | |
|
| |
| LM | +/-rhabdomyoblasts (eccentric nucleus, moderate amount of intensly eosinophilic cytoplasm, striations - not common); alveolar RMS: alveolus-like pattern (classic); embryonal RMS: small round cell tumour |
| Subtypes | embryonal (spindle cell subtype, botryoid), alveolar (translocation-positive, translocation-negative), undifferentiated |
| LM DDx | small round cell tumours - esp. small cell carcinoma and (large cell) lymphomas |
| IHC | desmin (best marker) +ve, actin +ve, myogenin +ve, CD56 +ve (common), synaptophysin -ve/+ve, chromogranin -ve/+ve, cytokeratins -ve/+ve |
| EM | sarcomeric like structures - typically in U-shaped cells |
| Molecular | alveolar RMS (~85% of cases): t(2,13) PAX3/FKHR fusion gene or t(1,13) PAX7/FKHR fusion gene |
| Site | soft tissue - skeletal muscle site (alveolar RMS), non-skeletal muscle site (embryonal RMS) |
|
| |
| Clinical history | alveolar RMS: young adult or adolescent; embryonal RMS: typically <10 years old |
| Prevalence | not common |
| Clin. DDx | other soft tissue tumours |
Rhabdomyosarcoma, often abbreviated RMS, is a malignant tumour of skeletal muscle.
General
- Most common paediatric sarcoma.
- Classically in the head and neck region.[1]
- Most common sarcoma in Li-Fraumeni syndrome.[2]
- ~6% of all childhood cancer.
Classification
Histologic
- Alveolar rhabdomyosarcoma.
- Usually young adults/adolescents.
- Early mets common.
- Usually arises in regions with skeletal muscle.
- Embryonal rhabdomyosarcoma.
- Usual <10 years old.
- Typically locally invasive.
- Usually arises in regions without skeletal muscle.
Less common types:[3]
- Undifferentiated rhabdomyosarcoma.
- Botryoid - may be considered a subtype of embryonal RMS.
- Spindle cell - may be considered a subtype of embryonal RMS.
Notes:
- How to remember the special types BUS: botryoid, undifferentiated, spindle.
- The above is the international classification. Several classification of RMS exist - see: Classifications of Rhabdomyosarcoma.[4]
Molecular and histologic
- Translocation-positive alveolar RMS.
- Translocation-negative alveolar RMS.
- Embryonal RMS.
Notes:
- Translocation-negative alveolar RMS shares gene expression profiling characteristics with embryonal RMS -- suggesting these can be grouped together.
Gross
Sarcoma botryoides (embryonal RMS) - distinctive appearance:
- Grapes on the vine-like clusters.
- Found in urinary bladder, vagina.
Image:
Microscopic
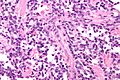
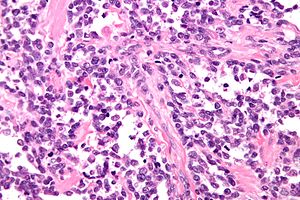
Alveolar rhabdomyosarcoma
Features:[2]
- Alveolus-like pattern -- key low-power feature.
- Fibrous septae lined by tumour cells.
- Cells may "fall-off" the septa, i.e. be detached/scattered in the alveolus-like space.
- Space between fibrous sepate may be filled with tumour = solid variant of alveolar rhabdomyosarcoma.
- Fibrous septae lined by tumour cells.
- Rhabdomyoblasts - essentially diagnostic.
- Eccentric nucleus.
- Moderate amount of intensly eosinophilic cytoplasm.
- Striations -- if you're really lucky; these are not common.
Other features:
- Nuclear pleomorphism - common.
- Mitoses - common.
Notes:
- Well-differentiated rhabdomyoblasts are uncommon in alveolar RMS.
DDx:
- Alveolar soft part sarcoma.
- Skeletal muscle regeneration.[5]
Images
www:
Embryonal rhabdomyosarcoma
Features:[2]
- Randomly arranged small cells.
- Myxoid matrix.
- Strap cells:
- Tadpole-like morphology.
- Rhabdomyoblasts - essentially diagnostic.
- Eccentric nucleus.
- Moderate amount of intensly eosinophilic cytoplasm.
- Striations -- if you're really lucky; these are not common.
DDx:
Images:
Subtypes of embryonal RMS
There are two common subtypes of embryonal RMS. Both of them have a better prognosis that embryonal RMS not otherwise specified (NOS).
Common subtypes:
- Botryoid subtype (AKA sarcoma botryoides):
- Gross: Grape-like morphology.
- Microscopic: Non-proliferating layer deep to the surface ("Cambium layer").
- Spindle cell subtype.
- General: may mimic leiomyosarcoma (complete with vesicular pattern) -- which is not common in the pediatric population.
- Microscopic: vesicular growth pattern, spindle cells.
Notes:
- Cambium layer = cellular region deep to epithelial component.[7]
- Can be thought of as the opposite of a "Grenz zone" -- which is a paucicellular zone between tumour and epithelium.
Anaplasia
Criteria:
- Hyperchromatic nuclei with size variation greater or equal to 3x.
- Multipolar (atypical) mitotic figures.
Subclassification:
- Focal - a few cells.
- Diffuse - cluster or sheets of anaplasia.
Notes:
- Not subtle - can identify at low power.
- Seen in 10-15% of RMS.
- More common in older individuals.
- Poorer prognosis in embryonal RMS.
- No change in prognosis in alveolar RMS.
IHC
Panel of muscle markers -- DAM:
- Desmin (best marker).
- Actin.
- Myogenin.
For head and neck RMS:[8]
- CD56 +ve.
- Synaptophysin -ve/+ve (seen in 12 of 37 cases[8]).
- Chromogranin A -ve/+ve (seen in 8 of 36 cases[8]).
- Wide-spectrum cytokeratin -ve/+ve.
- CAM5.2 -ve/+ve.
For urinary bladder RMS in adults:
- Myogenin +ve.
- Desmin +ve.
- Keratins -ve.[9]
- Keratin positive tumours are considered rhabdomyosarcomatous sarcomatoid carcinoma or sarcomatoid carcinoma with rhabdomyosarcomatous differentiation.
Subtyping via IHC
PST proposes[2] the following (presumably based on Makawitz et al.[10]):
| IHC | Translocation positive alveolar RMS |
Embryonal RMS | Translocation negative alveolar RMS |
| myogenin | +ve -- diffuse | +ve -- focal | +ve -- diffuse |
| EGFR | -ve | +ve | -ve |
| P-cadherin | +ve | -ve | -ve |
| IGF2 | -ve | +ve | +ve |
A paper by Wachtel at al.[11] proposes the use of:
- AP2beta and P-cadherin +ve in translocation positive alveolar RMS, and
- EGFR and fibrillin-2 +ve in embryonal RMS and translocation negative alveolar RMS.
Electron microscopy
Features:
- Sarcomeric like structures - usually in "bent" cells; cells that are U-shaped.
Molecular diagnostics
Alveolar rhabdomyosarcoma
Common translocations (~85% of cases):
- t(1,13).
- PAX7/FKHR fusion gene.
- Seen in approx. 15% of cases.
- t(2,13).[12]
- PAX3/FKHR fusion gene.
- Seen in approx. 70% of cases.
Notes:
- t(1,13) vs. t(2,13) -- t(1,13) usually: younger age, extremity lesion, localized disease, better survival.
- Several uncommon translocations exist.
- Important for urinary bladder lesions in adults: the presence of a translocation is more-or-less required for the diagnosis of RMS.[9]
- It is suggested that keratin negative tumours without molecular testing to corroborate the impression of RMS be referred to as rhabdomyomatous tumours.[9]
Embryonal rhabdomyosarcoma
- Chromosome 11p loss of heterozygosity.[13]
Note:
- Not used for diagnosis.
See also
References
- ↑ Rosenthal, TC.; Kraybill, W. (Aug 1999). "Soft tissue sarcomas: integrating primary care recognition with tertiary care center treatment.". Am Fam Physician 60 (2): 567-72. PMID 10465231.
- ↑ 2.0 2.1 2.2 2.3 Thorner, Paul S. 14 February 2011. Cite error: Invalid
<ref>tag; name "PST14feb11" defined multiple times with different content Cite error: Invalid<ref>tag; name "PST14feb11" defined multiple times with different content Cite error: Invalid<ref>tag; name "PST14feb11" defined multiple times with different content - ↑ Hicks, J.; Flaitz, C. (Jul 2002). "Rhabdomyosarcoma of the head and neck in children.". Oral Oncol 38 (5): 450-9. PMID 12110339.
- ↑ Parham, DM. (May 2001). "Pathologic classification of rhabdomyosarcomas and correlations with molecular studies.". Mod Pathol 14 (5): 506-14. doi:10.1038/modpathol.3880339. PMID 11353062.
- ↑ Guillou, L.; Coquet, M.; Chaubert, P.; Coindre, JM. (Aug 1998). "Skeletal muscle regeneration mimicking rhabdomyosarcoma: a potential diagnostic pitfall.". Histopathology 33 (2): 136-44. PMID 9762546.
- ↑ Chen, S.; Wang, S.; Gao, J.; Zhang, S. (May 2010). "[Pleuropulmonary blastoma: a clinicopathological analysis].". Zhongguo Fei Ai Za Zhi 13 (5): 550-3. doi:10.3779/j.issn.1009-3419.2010.05.31. PMID 20677658.
- ↑ URL: http://www.medilexicon.com/medicaldictionary.php?t=48297. Accessed on: 9 August 2011.
- ↑ 8.0 8.1 8.2 Bahrami, A.; Gown, AM.; Baird, GS.; Hicks, MJ.; Folpe, AL. (Jul 2008). "Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall.". Mod Pathol 21 (7): 795-806. doi:10.1038/modpathol.2008.86. PMID 18487991.
- ↑ 9.0 9.1 9.2 Bing, Z.; Zhang, PJ. (2011). "Adult urinary bladder tumors with rhabdomyosarcomatous differentiation: clinical, pathological and immunohistochemical studies.". Diagn Pathol 6: 66. doi:10.1186/1746-1596-6-66. PMID 21762516.
- ↑ Makawita S, Ho M, Durbin AD, Thorner PS, Malkin D, Somers GR (2009). "Expression of insulin-like growth factor pathway proteins in rhabdomyosarcoma: IGF-2 expression is associated with translocation-negative tumors". Pediatr. Dev. Pathol. 12 (2): 127–35. doi:10.2350/08-05-0477.1. PMID 18788888.
- ↑ Wachtel M, Runge T, Leuschner I, et al. (February 2006). "Subtype and prognostic classification of rhabdomyosarcoma by immunohistochemistry". J. Clin. Oncol. 24 (5): 816–22. doi:10.1200/JCO.2005.03.4934. PMID 16391296.
- ↑ URL: http://www.ncbi.nlm.nih.gov/omim/606597. Accessed on: 18 August 2010.
- ↑ Gallego Melcón, S.; Sánchez de Toledo Codina, J. (Jul 2007). "Molecular biology of rhabdomyosarcoma.". Clin Transl Oncol 9 (7): 415-9. PMID 17652054.