Melanocytic lesions
Melanocytic lesions are commonly encountered in dermatopathology and an area which causes some difficulty, i.e. it is hard to decide in some cases whether a lesion is benign (e.g. Spitz nevus) or malignant (malignant melanoma).
Overview
Identifying melanocytes
- Clear or pigmented cytoplasm.
- +/-Nuclear pseudoinclusions.
- Epithelioid (superficial) or spindled (deep).
Benign lesions
| Name | Key feature | Microscopic | Clinical | Gross | Image | Ref. |
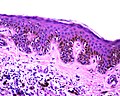
| Lentigo simplex | no nests, epidermis only | slender rete with melanocytes; no nests of melanocytes; no dermal melanocytes | < 40 years | small flat pigmented lesion | [1] | |
| Junctional melanocytic nevus | nests in epidermis | nests of melanocytes at tips of rete, no dermal melanocytes | usu. sun exposed skin, unusual in >50 years | small flat (uniformly) pigmented lesion | [1] | |
| Compound melanocytic nevus | benign nests in dermis & epidermis | nests of melanocytes at tips of rete and in dermis; dermal melanocytes lack nucleoli, lack mitoses and "mature with depth" -- see Note 1. | small slightly raised (uniformily) pigmented lesion | [2] | ||
| Intradermal melanocytic nevus | nested & individual melanocytes - only in dermis | nested & individual melanocytes - only in dermis, +/- multinucleation, +/-pseudovascular spaces | Clinical DDx: fibroepithelial polyp (skin tag), basal cell carcinoma | raised, non-pigmented lesion | [2] | |
| Spitz nevus (epithelioid and spindle-cell nevus) | long axis of nests perpendicular to surface, DE junction lesion | spindled, epithelioid or mixed melanocytes, long axis of nests perpendicular to surface, superficial mitoses common, +/-hyperkeratosis, +/-acanthosis, +/-hypergranulosis | Children & adolescents | usu. non-pigmented | Spitz nevus (drmihm.com), Spitz nevus - high mag. (WC), Spitz nevus - intermed. mag. (WC) | [2] |
| Pigmented spindle cell nevus of Reed (AKA Pigmented spindle cell nevus) | nests of heavily pigmented spindle cells, DE junction lesion | heavily pigmented spindle cells in epidermis & dermis, form "basket weave" pattern, well-circumscribed | women in teens & 20s; location: shoulder, pelvic girdle region | Pigmented +++, small size | Reed nevus - low mag. (WC), Reed nevus - intermed. mag. (WC), Reed nevus - collection (histopathology-india.net) | [3] |
| Blue nevus | lentil-shaped (ovoid) nests btw collagen bundles, dermal lesion | lentil-shaped nests, mix of spindle or dendritic or epithelioid cell morphology, nests btw collagen | usu. head & neck or extremities; clinically confused with melanoma[4] | blue flat or slightly raised lesion | Blue nevus (WC), Blue nevus - very low mag. (WC), Blue nevus - intermed. mag. (WC) | [5] |
| Cellular blue nevus | dermal lesion with pigmented spindle cells & epithelioid cells | deep dermis +/-subcutis extension; cells lack nucleoli; biphasic: (1) epithelioid cells with pale cytoplasm, (2) pigmented spindle cells +/- melanophages | congenital or acquired; usu. scalp or butt | blue flat or raised lesion | [5] | |
| Congenital-pattern nevus | growth along dermal structures | extend along dermal structures (e.g. nerves, hair shafts, ducts); lacks atypia; +/-mitoses | congenital or acquired; large ones increased melanoma risk[6] | small, intermediate (2-20 cm) or large | [5] | |
| Dysplastic nevus (Clark's nevus) | melanocyte bridges, lamellar fibrosis | melanocytes "bridges" between sides of rete ridges, "lamellar fibrosis" (collagen deep to epidermis), mod. atypia | may be familial - precursor to melanoma | may have asymmetry in shape or pigmentation | Dysplastic nevus - low mag., Dysplastic nevus - high mag. | [7] |
| Halo nevus | lymphocytes +++ | lymphocytes at perimeter of melanocytic; epidermal melanocytes not nested; may be dermal, epidermal or both | central zone of pigment | [7] |
Note 1:
- "Maturation" in the context of melanocytic lesions means (1) the cells get smaller with depth, (2) cells are less mitotic with depth.
Lentiginous melanocytic lesions
Lentigo simplex
General
- Benign.
- Usually <40 years old.
Fits into the larger category of lentiginous melanocytic proliferations - these include:[9]
- Solar lentigo.
- Lentigo simplex.
- Lentiginous nevus.
- Lentiginous melanoma in situ.
Associated syndromes:[10]
Gross
- Small flat pigmented lesion.[1]
DDx - clinical:
Microscopic
Features:[1]
- Melanocytes in epidermis only.
- Melanocytes basally located (normal location) with hyperpigmentation.
- No melanocytic nests.
- +/-Mild/moderate elongation of the rete ridges.[11]
DDx:[12]
- Solar lentigo - in sun exposed areas.
- Ephelis (freckle) - change with UV light exposure.
- Melanotic macule.
- Lentiginous nevus.
Images:
Sign out
SKIN LESION, LEFT ABDOMEN, BIOPSY: - SIMPLE LENTIGO, COMPLETELY EXCISED IN THE PLANE OF SECTION.
SKIN LESION, LEFT ABDOMEN, BIOPSY: - BENIGN SIMPLE LENTIGO.
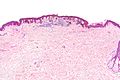
Solar lentigo
- Plural solar lentigines.
General
- Benign.
Fits into the larger category of lentiginous melanocytic proliferations - these include:[9]
- Solar lentigo.
- Lentigo simplex.
- Lentiginous nevus.
- Lentiginous melanoma in situ.
Gross
Features:[9]
- Small (< 4 mm), irregular brown macule.
- Usu. sun-exposed area.
DDx (clinical):
- Lentigo maligna - melanoma in situ on sun damaged skin.
- Seborrheic keratosis, flat.[13]
Microscopic
Features:[14]
- Hyperpigmented melanocytes - may be present in increased quantities - key feature.
- Classically at the tips of the rete ridges.
- No (melanocyte) nuclear atypia.
- Solar damage (solar elastosis).[11]
Notes:
- Should not be present:[9]
- Nests of melanocytes.
- Pagetoid spread of melanocytes.
DDx:[14]
- Pigmented actinic keratosis.
- Lichen planus-like keratosis.
- Pigmented seborrheic keratosis.
Images:
Sign out
FOREARM LESION, RIGHT, PUNCH BIOPSY: - SOLAR LENTIGO.
Micro
The sections show hair-bearing skin with basilar pigmentation at the tips of the rete ridges and solar elastosis. No melanocytic nests are identified. The epidermal cells mature to the surface. No significant inflammation is present.
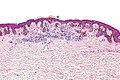
Lentiginous melanocytic nevus
- AKA lentiginous nevus.
General
- Benign.
Fits into the larger category of lentiginous melanocytic proliferations - these include:[9]
- Solar lentigo.
- Lentigo simplex.
- Lentiginous nevus.
- Lentiginous melanoma in situ.
Gross
- Small flat pigmented lesion.
Microscopic
Features:[9]
- Melanocytes without atypia in the epidermis only - key feature.
- Melanocytic nests.
- +/-Rete ridge elongation.
DDx:
- Atypical lentiginous nevus.
- Dysplastic nevus.
- Melanoma in situ - especially if solar elastosis is present.
Images
www:
Sign out
SKIN LESION, BACK, SHAVE EXCISION: - JUNCTIONAL LENTIGINOUS NEVUS. -- COMPLETELY EXCISED (LATERAL CLEARANCE 1 MM).
Micro
The sections show skin with non-nested melanocytes in the epidermis. The melanocytes have no significant cytologic atypia. There is no upward scatter of melanocytes. The lesion is completely excised the in plane of section.
Nested melanocytic lesions
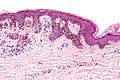
Common melanocytic nevus
- AKA common nevus.
- In common language: mole.
General
- Benign.
- Common.
- Think melanoma.
Clinical:
- ABCD = asymmetric, borders (irregular), colour (black), diameter (large).
Microscopic
Features:
- Symmetrical lesion.
- "Matures" with depth
- Less cellular with depth
- Less nuclear atypia with depth.
- Smaller cells with depth.
- Smaller nests with depth.
- Rare mitoses (superficial).
- No deep mitoses.
- No destruction of surrounding structures.
- No nucleoli.
Subtypes
Compound melanocytic nevus
- Abbreviated CMN and CN.
- In the dermis and epidermis - key feature.
Junctional melanocytic nevus
- Abbreviated JN.
- In the epidermis - key feature.
Intradermal melanocytic nevus
- Only in the dermis - key feature.
- +/-Adipocytes - uncommon.[16]
Note:
- In the oral cavity these are known as intramucosal nevi.
Sign out
Junctional melanocytic nevus
SKIN LESION, RIGHT LATERAL UPPER ARM, BIOPSY: - BENIGN JUNCTIONAL NEVUS.
Micro
The sections show melanocytes in nest confined to the epidermis. The lesion is symmetrical in its architecture and pigment distribution. There is no pagetoid spread of melanocytes in the epidermis. No significant nuclear atypia is identified. No mitotic activity is appreciated.
The lesion is completely excised in the plane of section.
Compound melanocytic nevus
SKIN LESION, RIGHT NECK, SHAVE BIOPSY: - BENIGN COMPOUND NEVUS.
Micro
The sections show melanocytes in the dermis and epidermis. The lesion is symmetrical in its architecture and pigment distribution. There is no pagetoid spread of melanocytes in the epidermis. Superficially, melanocytes are in nests. Melanocytes show no apparent cytologic atypia and mature with depth. No mitotic activity is appreciated.
The lesion is completely excised in the plane of section.
Barely compound
The sections show melanocytes predominantly in the dermis. Rare melanocytic nests are seen at the dermal-epidermal junction. There is no pagetoid spread of melanocytes in the epidermis. The lesion is symmetrical in its architecture. Superficially, melanocytes are in nests and pigment is present. The melanocytes mature with depth. No mitotic activity is appreciated.
The lesion is present at the margin.
Intradermal melanocytic nevus
SKIN LESION, BACK, PUNCH BIOPSY: - BENIGN INTRADERMAL NEVUS.
Adipocytes present
SKIN LESION, BACK, PUNCH BIOPSY: - BENIGN INTRADERMAL NEVUS WITH MATURE ADIPOCYTES.
Clinically suspicious
SKIN LESION, LEFT UPPER ARM, PUNCH BIOPSY: - BENIGN INTRADERMAL NEVUS, INCOMPLETELY EXCISED. COMMENT: HMB-45 marks very rare superficial cells. Ki-67 staining does not show apparent melanocyte staining. MITF marks the melanocytes and demonstrates maturation of the melanocytes with depth.
Micro
The sections show melanocytes in the dermis separated from the epidermis by a Grenz zone. The lesion is symmetrical in its architecture and pigment distribution. Superficially, melanocytes are in nests. Melanocytes mature with depth. No mitotic activity is appreciated.
The lesion is completely excised in the plane of section.
Congenital-pattern nevus
- AKA congenital nevus.
General
- Congenital or acquired - thus "congenital-pattern".
- Large ones increase melanoma risk.[6]
- Small (<2 cm), intermediate (2-20 cm), large (>20 cm).
Microscopic
Features:[5]
- Growth along dermal structures - key feature.
- Nerves, hair shafts, ducts.
- "Deep" melanocytes.
- Lacks nuclear atypia.
- +/-Mitoses.
- +/-Less nesting.[17]
DDx:
Images:
Sign out
SKIN LESION, LEFT UPPER BACK, BIOPSY: - COMPOUND MELANOCYTIC NEVUS WITH CONGENITAL FEATURES.
Recurrent melanocytic nevus
General
- Classically, arises at the site of a partially excised nevus.[18]
Microscopic
Features - three layers (often described as a "sandwich"):
- Features of a compound nevus or junctional nevus.
- Scar.
- Thick collagen bundles arranged parallel to the skin surface.
- Features of an intradermal nevus.
May have:[18]
- Pagetoid spread of melanocytes.
- Confluent nests at the DE junction.
- Nuclear atypia - uncommon.
DDx:[18]
- Melanoma in situ - classically spread beyond the scar, unlike pseudomelanoma - growth confined to epidermis.
- Sclerosing nevus.
IHC
- HMB-45 deep component -ve.
- Ki-67 - non-proliferative.
Sclerosing melanocytic nevus
- AKA sclerosing nevus.
General
- Similar to recurrent nevus.
- Clinically may be confused with melanoma.
- No history of trauma or prior excision.
Microscopic
Features:[19]
- Trizonal - may be described as a sandwich:
- Junctional or compound nevus.
- May have pagetoid spread of melanocytes, i.e. non-basal melanocytes in the epidermis.
- Cannot have cytologic atypia - presence of cytologic atypia in this layer makes it melanoma.
- Fibrotic tissue with irregular melanocytic nests.
- Nevus below scar - may be common, congenital-type, dysplastic, Spitz.
- Junctional or compound nevus.
DDx:
- Malignant melanoma.
- Recurrent melanocytic nevus - also trizonal; however, has a fibrotic layer that does not have melanocytes in it.[19]
Pigmented spindle cell nevus
- AKA pigmented spindle cell nevus of Reed.
General
- Uncommon.
- Women in teens & 20s.
- Location: shoulder, pelvic girdle region.
Microscopic
Features:[3]
- Nests of heavily pigmented spindle cells at dermal-epidermal junction - key feature.
- Nevoid cells in epidermis & dermis - form "basket weave" pattern
- Well-circumscribed lesion.
Notes:
- No epithelioid nevus cells.
DDx:
Images:
- WC:
- www:
Spitz nevus
- AKA epithelioid and spindle-cell nevus.
General
- May be very difficult to differentiate from melanoma.
Epidemiology:
- Children & adolescents.
Treatment:
- Complete excision.[20]
Gross
- Usually face or extremity.[21]
Microscopic
Features:[2]
- Architecture:
- Nests of cells (spindle, epithelioid or spindle/epithelioid) - in both dermis and epidermis.
- Nests are vertically arranged, i.e. the long axis of the nests are perpendicular to the skin surface.
- Nest arrangement/orientation described as "cluster of bananas".
- Nests are vertically arranged, i.e. the long axis of the nests are perpendicular to the skin surface.
- Nests of cells (spindle, epithelioid or spindle/epithelioid) - in both dermis and epidermis.
- +/-Hyperkeratosis (more keratin, i.e. thick stratum corneum).
- +/-Hypergranulosis (thick stratum granulosum).
- +/-Acanthosis (thick stratum spinosum).
- Kamino bodies (also written Camino bodies) - dense eosinophilic bodies.[22]
- Apoptotic cells.
- Kamino bodies are rare in melanoma.
Notes:
- Never in the setting of solar elastosis.[23]
- If there is solar elastosis -- it's melanoma.
DDx:
Images:
- www:
- WC:
Acral nevus
General
- Palms or soles.
Note:
- Volar refers to the palmar aspect or plantar aspect.[24]
Gross
- Pigmented lesion.
Note:
- Should be bisected perpendicular to the dermatoglyphs (ridges).[25]
- Lesions sectioned parallel to the ridges (on microscopy) may appear to have confluent junctional nests (leading to the diagnosis of melanoma).
Microscopic
Features:
- Acral skin:
- Thick stratum corneum (hyperkeratotic).
- Thick stratum spinosum (acanthotic).
- Nevus with intraepidermal ascent of cells.
- May be referred to as Pagetoid scatter.
- Should not be present at the edge of the lesion - key feature.[25]
Notes:
- Intraepidermal ascent of cells is usually suggestive of melanoma.
- In acral sites (esp. at the centre of a lesion) the criteria are relaxed, i.e. this is considered benign for this site.
- Nests in the ridges raise the suspicion of melanoma.
- Memory device "ridges are risky, furrows are fine".[25]
DDx:
- Acral lentiginous melanoma.
Dysplastic melanocytic nevus
- AKA dysplastic nevus.
- AKA Clark nevus.
- AKA nevus with architectural disorder.
- This term is recommended by the American NIH; however, it is not widely adopted.[25]
General
- Benign.
- Dysplastic nevi are considered a risk factor for melanoma and may be a precursor of melanoma, as the name dysplastic nevus suggests.[26]
- Most melanoma cases do not have evidence of a pre-existing (dysplastic) nevus.[27]
- Large numbers of these nevi (10-100) are seen in dysplastic nevus syndrome.[28]
- Individuals with this syndrome have an increased risk of melanoma and approximately one third of their melanomas arise from a Clark nevus. The other two thirds arise de novo.
Clinical:[29]
- Associated with sun exposure.
Treatment:
- Dysplastic nevi with severe nuclear atypia are treated like melanoma - excision with a 5 mm margin.
- Dysplastic nevi with moderate nuclear atypia with margin involvement are re-excised.[30]
Gross
Features:[29]
- "Ugly duckling sign" - lesion looks different than the rest.
- ABCDE criteria of in melanoma.
- Tend to be greater than 5 mm - important.† [31]
Note:
- † Size matters - things smaller than 4 mm are usually not a dysplastic nevus.
Microscopic
Features:[7]
- Melanocytes "bridges" between sides of rete ridges.
- Joining of three or more adjacent rete ridges = suspicious for melanoma.[30]
- Drapping fibrous tissue - "lamellar fibrosis" - collagen deep to epidermis.
- Usually moderate nuclear atypia (small nucleoli present) - see grading section.
- +/-Junctional component (cells in the epidermis at the DE junction) larger than the intradermal component - shoulder phenomenon.
DDx:
- Melanoma in situ - especially on sun damaged skin (lentigo maligna).[32][33]
- Malignant melanoma - especially on sun damaged skin (lentigo maligna melanoma).
- Compound melanocytic nevus.
- Atypical lentiginous nevus.
Images
Grading
These lesions are often graded § :[34]
| Grade | Size of nucleus | Other features |
|---|---|---|
| Mild | 1x keratinocyte nucleus | no nucleoli/very small nucleoli †,[35] slight hyperchromasia |
| Moderate | 1-2x keratinocyte nucleus | small nucleoli †, irregular nuclear contours |
| Severe | >2x keratinocyte nucleus | prominent nucleoli †‡ |
† The sizes "very small", "small" and "prominent" are not defined; it is suggested that "very small" is visible with the 40x objective, "small" with the 20x objective and "prominent" with the 10x objective. Focal, rare small nucleoli are not significant; they can be seen in benign melanocytic nevi.
‡ Prominent nucleoli (alone) is considered enough to call "severe".[34]
Notes:
- § There is no consensus on this and practise (embarrassingly) is all over the map.[25]
- A two-tier grading system also exists (low-grade (leave it alone) / high-grade (cut it out)).
- Some pathologists do not grade dysplastic nevi.
Sign out
Compound
SKIN LESION, BACK, EXCISION: - DYSPLASTIC COMPOUND NEVUS WITH MILD CYTOLOGIC ATYPIA AND MILD ARCHITECTURAL ATYPIA, COMPLETELY EXCISED IN THE PLANE OF SECTION (2 MM CLEARANCE).
SKIN LESION, CENTRAL BACK, EXCISION: - DYSPLASTIC COMPOUND NEVUS WITH MILD CYTOLOGIC ATYPIA. - NEAREST MARGIN (LATERAL MARGIN) 6 MM.
Micro
The sections show hair-bearing skin with a compound melanocytic lesion. The epidermal component extends at least three rete ridges further than the dermal component (shoulder phenomenon). There is bridging between the sides of the rete ridges and lamellar fibrosis. The melanocyte nuclei are approximately the size of the keratinocyte nuclei, and do not have a prominent nucleolus (mild cytologic atypia).
There is no upward scatter of melanocytes and melanocytes in the dermis are mature. No mitotic activity is appreciated.
Junctional
SKIN LESION, LOWER BACK, EXCISION: - DYSPLASTIC JUNCTIONAL MELANOCYTIC NEVUS WITH MILD NUCLEAR ATYPIA. - NEAREST MARGIN (LATERAL MARGIN) 1 MM.
Micro
The sections show hair-bearing skin with a junctional melanocytic lesion. There is bridging between the sides of the rete ridges and lamellar fibrosis. The melanocyte nuclei are approximately the size of the keratinocyte nuclei, and do not have a prominent nucleolus (mild nuclear atypia).
There is no upward scatter of melanocytes. No mitotic activity is appreciated.
Desmoplastic melanocytic nevus
- AKA sclerosing melanocytic nevus.
General
- Benign.
Gross
- Usu. "small" and symmetric.
Clinical DDx:
Microscopic
Features:[36]
- Compound nevus or intradermal nevus with prominent dermal fibrosis.
- Fibrosis: extra pink - versus surrounding.
DDx:
IHC
Features:[36]
- Melan A (MART-1) +ve (not usu. +ve in desmoplastic melanoma).[36]
- S-100 +ve.
- Ki-67 -- only rare cells.
Miscellaneous
Blue nevus
- Common blue nevus redirects here.
- Abbreviated BN.
General
- Usually head & neck, extremities (e.g. dorsum of wrist or foot) or buttock.[37]
- Clinically confused with malignant melanoma.[4]
- Second most common melanocytic lesion of the oral cavity.[38]
- Most common melanocytic lesion intramucosal melanocytic nevus.
Gross
- Blue flat or slightly raised lesion.
Microscopic
Features:[5]
- Lentil-shaped (ovoid) nests between collagen bundles.
- Mix of spindle or dendritic or epithelioid cell morphology.
Notes:[39]
- Classically no epidermal component.
- The entity compound blue nevus has been described; it is rare.[40]
- May be assoc. with a hair follicle.
DDx:
- Dermatofibroma - esp. amelanotic BN.
- Malignant melanoma.
- Clear cell sarcoma.
- Combined melanocytic nevus - blue nevus found together with another nevus (classically common melanocytic nevus), superficial.
- Pigmented epithelioid melanocytoma - superificial dermis, has nuclear atypia.
- Vascular lesions (venous lake, hemangioma).
Images:
Variants of blue nevus
Several histologic variants:[39]
- Common blue nevus - the blue nevus not otherwise specified.
- Cellular blue nevus.
- Amelanotic blue nevus - may be confused with a dermatofibroma.
- Sclerosing blue nevus - has stromal fibrosis.
- Epithelioid blue nevus.
Memory device:
- C CASE = Common, Cellular, Amelanotic, Sclerosing, Epithelioid.
Sign out
SKIN LESION, RIGHT WRIST DORSUM, PUNCH BIOPSY: - COMMON BLUE NEVUS.
SKIN LESION, LEFT SHIN, PUNCH BIOPSY: - SCLEROSING BLUE NEVUS.
Micro
The sections show pigmented spindle cells in the dermis between collagen bundles. The spindle cells show no nuclear atypia and no mitotic activity is appreciated.
The lesion does not extend into the epidermis. There are no melanocyte nests. There is no significant inflammation. The lesion is completely excised.
The pigmented cells are negative on the Prussian blue stain.
Cellular blue nevus
General
- Congenital or acquired.
- Usually scalp or butt.
- Variant of the common blue nevus.
Microscopic
Features:[5]
- Dermal lesion with pigmented spindle cells & epithelioid cells - key feature.
- Cells lack nucleoli.
- Biphasic:
- Epithelioid cells with pale cytoplasm.
- Pigmented spindle cells +/- melanophages.
Images:
Combined melanocytic nevus
- AKA combined nevus.
General
- Uncommon.
- Morphologic features of two types of melanocytic nevi.
- Most common: blue nevus and common nevus.[41]
Microscopic
Features:[41]
- Morphologic features of two types of melanocytic nevi.
DDx:
- Malignant melanoma.
- Pigmented epithelioid melanocytoma - superificial dermis, has nuclear atypia.
Sign out
A. SKIN LESION, LEFT UPPER ARM, PUNCH BIOPSY: - BENIGN COMBINED MELANOCYTIC NEVUS (INTRADERMAL MELANOCYTIC NEVUS AND BLUE NEVUS).
Neurocristic hamartoma
See also
References
- ↑ 1.0 1.1 1.2 1.3 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 498. ISBN 978-0781765275.
- ↑ 2.0 2.1 2.2 2.3 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 499. ISBN 978-0781765275.
- ↑ 3.0 3.1 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 500. ISBN 978-0781765275.
- ↑ 4.0 4.1 Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 592. ISBN 978-1416054542.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 501. ISBN 978-0781765275.
- ↑ 6.0 6.1 Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1170. ISBN 978-1416031215.
- ↑ 7.0 7.1 7.2 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 502. ISBN 978-0781765275.
- ↑ URL: http://www.dermnetnz.org/lesions/lentigo-simplex.html. Accessed on: 27 March 2013.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 438. ISBN 978-0443066542.
- ↑ 10.0 10.1 URL: http://dermaamin.com/site/histopathology-of-the-skin/64-l/1852-lentigo-simplex-.html. Accessed on: 17 December 2012.
- ↑ 11.0 11.1 11.2 Hafner, C.; Stoehr, R.; van Oers, JM.; Zwarthoff, EC.; Hofstaedter, F.; Klein, C.; Landthaler, M.; Hartmann, A. et al. (Nov 2009). "The absence of BRAF, FGFR3, and PIK3CA mutations differentiates lentigo simplex from melanocytic nevus and solar lentigo.". J Invest Dermatol 129 (11): 2730-5. doi:10.1038/jid.2009.146. PMID 19536147.
- ↑ 12.0 12.1 URL: http://www.humpath.com/?lentigo-simplex. Accessed on: 17 December 2012.
- ↑ 13.0 13.1 URL: http://www.dermaamin.com/site/histopathology-of-the-skin/53-a/1555-actinic-lentigo-.html. Accessed on: 6 May 2013.
- ↑ 14.0 14.1 Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 437. ISBN 978-0443066542.
- ↑ Fullen, DR.; Reed, JA.; Finnerty, B.; McNutt, NS. (Sep 2001). "S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi.". J Cutan Pathol 28 (8): 393-9. PMID 11493376.
- ↑ Eng, W.; Cohen, PR. (Nov 1998). "Nevus with fat: clinical characteristics of 100 nevi containing mature adipose cells.". J Am Acad Dermatol 39 (5 Pt 1): 704-11. PMID 9810886.
- ↑ 17.0 17.1 URL: http://www.dermpedia.org/dermpedia-textbook/congenital-nevus. Accessed on: 27 December 2012.
- ↑ 18.0 18.1 18.2 Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 465. ISBN 978-0443066542.
- ↑ 19.0 19.1 Fabrizi, G.; Pennacchia, I.; Pagliarello, C.; Massi, G. (Nov 2008). "Sclerosing nevus with pseudomelanomatous features.". J Cutan Pathol 35 (11): 995-1002. doi:10.1111/j.1600-0560.2007.00941.x. PMID 18537860.
- ↑ Gelbard, SN.; Tripp, JM.; Marghoob, AA.; Kopf, AW.; Koenig, KL.; Kim, JY.; Bart, RS. (Aug 2002). "Management of Spitz nevi: a survey of dermatologists in the United States.". J Am Acad Dermatol 47 (2): 224-30. PMID 12140468.
- ↑ Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 449. ISBN 978-0443066542.
- ↑ Kirkwood, John M.; Jukic, Drazen; Averbook, Bruce J.; Sender, Leonard S. (October 2009). "Melanoma in Pediatric, Adolescent, and Young Adult Patients". Semin Oncol. 36 (5): 419-31. PMC 2797485. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2797485/.
- ↑ Jakubovic, H. 16 July 2010.
- ↑ URL: http://www.medterms.com/script/main/art.asp?articlekey=9907. Accessed on: 14 January 2013.
- ↑ 25.0 25.1 25.2 25.3 25.4 Elston, D. (Jul 2012). "Practical advice regarding problematic pigmented lesions.". J Am Acad Dermatol 67 (1): 148-55. doi:10.1016/j.jaad.2012.04.006. PMID 22703907.
- ↑ Rezze, GG.; Leon, A.; Duprat, J. (Dec 2010). "Dysplastic nevus (atypical nevus).". An Bras Dermatol 85 (6): 863-71. PMID 21308311.
- ↑ Longo, C.; Rito, C.; Beretti, F.; Cesinaro, AM.; Piñeiro-Maceira, J.; Seidenari, S.; Pellacani, G. (Sep 2011). "De novo melanoma and melanoma arising from pre-existing nevus: in vivo morphologic differences as evaluated by confocal microscopy.". J Am Acad Dermatol 65 (3): 604-14. doi:10.1016/j.jaad.2010.10.035. PMID 21715047.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 155600
- ↑ 29.0 29.1 Dediol, I.; Bulat, V.; Zivković, MV.; Marković, BM.; Situm, M. (Sep 2011). "Dysplastic nevus--risk factor or disguise for melanoma.". Coll Antropol 35 Suppl 2: 311-3. PMID 22220461.
- ↑ 30.0 30.1 Culpepper, KS.; Granter, SR.; McKee, PH. (Nov 2004). "My approach to atypical melanocytic lesions.". J Clin Pathol 57 (11): 1121-31. doi:10.1136/jcp.2003.008516. PMID 15509670.
- ↑ Barnhill, RL.; Hurwitz, S.; Duray, PH.; Arons, MS. (Feb 1988). "The dysplastic nevus: recognition and management.". Plast Reconstr Surg 81 (2): 280-9. PMID 3275948.
- ↑ Farrahi, F.; Egbert, BM.; Swetter, SM. (Jul 2005). "Histologic similarities between lentigo maligna and dysplastic nevus: importance of clinicopathologic distinction.". J Cutan Pathol 32 (6): 405-12. doi:10.1111/j.0303-6987.2005.00355.x. PMID 15953373.
- ↑ Edwards, SL.; Blessing, K. (Jun 2000). "Problematic pigmented lesions: approach to diagnosis.". J Clin Pathol 53 (6): 409-18. PMID 10911797.
- ↑ 34.0 34.1 Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 447-8. ISBN 978-0443066542.
- ↑ Arumi-Uria, M.; McNutt, NS.; Finnerty, B. (Aug 2003). "Grading of atypia in nevi: correlation with melanoma risk.". Mod Pathol 16 (8): 764-71. doi:10.1097/01.MP.0000082394.91761.E5. PMID 12920220.
- ↑ 36.0 36.1 36.2 Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 464. ISBN 978-0443066542.
- ↑ Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 456. ISBN 978-0443066542.
- ↑ URL: http://emedicine.medscape.com/article/1079272-overview. Accessed on: 10 December 2012.
- ↑ 39.0 39.1 Busam, Klaus J. (2009). Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series (1st ed.). Saunders. pp. 457. ISBN 978-0443066542.
- ↑ Kamino, H.; Tam, ST. (Oct 1990). "Compound blue nevus: a variant of blue nevus with an additional junctional dendritic component. A clinical, histopathologic, and immunohistochemical study of six cases.". Arch Dermatol 126 (10): 1330-3. PMID 2221938.
- ↑ 41.0 41.1 Baran, JL.; Duncan, LM. (Oct 2011). "Combined melanocytic nevi: histologic variants and melanoma mimics.". Am J Surg Pathol 35 (10): 1540-8. doi:10.1097/PAS.0b013e31822e9f5e. PMID 21881487.
External links
- Dear 16 year-old me (youtube.com) - with Dr. G.