Difference between revisions of "Medulloblastoma"
Jump to navigation
Jump to search
Jensflorian (talk | contribs) (→Genetically defined: update) |
Jensflorian (talk | contribs) |
||
| Line 6: | Line 6: | ||
*Mostly paediatric population. | *Mostly paediatric population. | ||
*May be seen as a component of [[nevoid basal cell carcinoma syndrome]] (NBCCS). | *May be seen as a component of [[nevoid basal cell carcinoma syndrome]] (NBCCS). | ||
** | **Show molecularly spatially homogeneous transcriptomes, allowing for accurate subgrouping of tumors from a single biopsy.<ref>{{Cite journal | last1 = Morrissy | first1 = AS. | last2 = Cavalli | first2 = FMG. | last3 = Remke | first3 = M. | last4 = Ramaswamy | first4 = V. | last5 = Shih | first5 = DJH. | last6 = Holgado | first6 = BL. | last7 = Farooq | first7 = H. | last8 = Donovan | first8 = LK. | last9 = Garzia | first9 = L. | title = Spatial heterogeneity in medulloblastoma. | journal = Nat Genet | volume = 49 | issue = 5 | pages = 780-788 | month = May | year = 2017 | doi = 10.1038/ng.3838 | PMID = 28394352 }}</ref> | ||
*Commonly spread via cerebrospinal fluid (CSF).<ref>{{Ref APBR|424 Q34}}</ref> | *Commonly spread via cerebrospinal fluid (CSF).<ref>{{Ref APBR|424 Q34}}</ref> | ||
**May be detected in [[CSF cytopathology]] specimens. | **May be detected in [[CSF cytopathology]] specimens. | ||
Revision as of 15:00, 21 September 2017
Medulloblastoma is a malignant small round cell tumour that is found in the cerebellum.
Morphologically identical supratentorial tumours are called primitive neuroectodermal tumour (PNET).
General
- Mostly paediatric population.
- May be seen as a component of nevoid basal cell carcinoma syndrome (NBCCS).
- Show molecularly spatially homogeneous transcriptomes, allowing for accurate subgrouping of tumors from a single biopsy.[1]
- Commonly spread via cerebrospinal fluid (CSF).[2]
- May be detected in CSF cytopathology specimens.
Gross
- Location: cerebellum - key feature.
- Morphologically identical supratentorial tumours are called primitive neuroectodermal tumour (PNET).
- Supratentorial and spinal metastases from initial tumor possible.
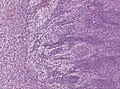
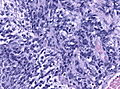
Microscopic
Features:[3]
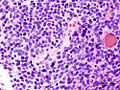
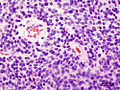
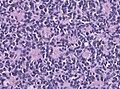
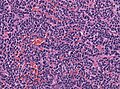
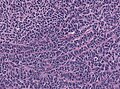
- Small round cell tumour.
- Homer-Wright rosettes:
- Rosette with a meshwork of fibers (neuropil) at the centre.[4]
IHC
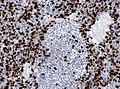
- MAP2 usu. +ve
- Synaptophysin +ve (weak to strong)
- NSE +ve/-ve
- NF +ve/-ve
- Chromogranin +ve/-ve
- GFAP +ve/-ve (mostly along blood vessels)
- Vimentin +ve
- Nestin +ve
- INI1 retained (no loss)
DDx:
Images
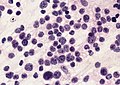
Case:
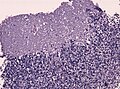
Partial MAP2 immunoreactivity. (WC/jensflorian)
www:
- Medulloblastoma (ouhsc.edu).
- Medulloblastoma - several images (upmc.edu).
- Medulloblastoma with rhabdomyoblastic differentiation - several images (upmc.edu).
Subtypes
Histologically defined
- Classic medulloblastoma (~85% of all medulloblastomas).
- Variants of medulloblastoma (~15% of all medulloblastomas together):
- Anaplastic / Large cell variant.
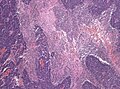
- Desmoplastic/nodular medulloblastoma (DNMB).
- Medulloblastoma with extensive nodularity (MBEN).
Anaplastic variant
Features:
- Larger cells.
- Severe anaplasia.
- Polygonal cells.
Genetically defined
- WNT (brainstem-related, usu. CTNNB1 mutations, less common: CSNK2B, AXIN2, APC).[5]
- SHH (PTCH1 & SUFU in infant, PTCH1, SMO, Gli2, MYCN in non-infant).[6]
- infantile and adult cases are biologically different, esp p53 mutant tumors are associated with poor outcome.[7]
- Group 3
- Group 4
Prognosis
- Prognosis based on histology:[8][9][10] DNMB & MBEN > classic > anaplastic & large cell variant.
- Prognosis based on genetics:[11] WNT > SHH (without Tp53 mut) > Group 4 > Group 3 > SHH (with Tp53 mut).
See also
References
- ↑ Morrissy, AS.; Cavalli, FMG.; Remke, M.; Ramaswamy, V.; Shih, DJH.; Holgado, BL.; Farooq, H.; Donovan, LK. et al. (May 2017). "Spatial heterogeneity in medulloblastoma.". Nat Genet 49 (5): 780-788. doi:10.1038/ng.3838. PMID 28394352.
- ↑ Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 424 Q34. ISBN 978-1416025887.
- ↑ URL: http://moon.ouhsc.edu/kfung/jty1/neurotest/Q93-Ans.htm. Accessed on: 26 October 2010.
- ↑ Wippold FJ, Perry A (March 2006). "Neuropathology for the neuroradiologist: rosettes and pseudorosettes". AJNR Am J Neuroradiol 27 (3): 488–92. PMID 16551982.
- ↑ Wefers, AK.; Warmuth-Metz, M.; Pöschl, J.; von Bueren, AO.; Monoranu, CM.; Seelos, K.; Peraud, A.; Tonn, JC. et al. (2014). "Subgroup-specific localization of human medulloblastoma based on pre-operative MRI.". Acta Neuropathol 127 (6): 931-3. doi:10.1007/s00401-014-1271-5. PMID 24699697.
- ↑ Neumann, JE.; Swartling, FJ.; Schüller, U. (Jul 2017). "Medulloblastoma: experimental models and reality.". Acta Neuropathol. doi:10.1007/s00401-017-1753-3. PMID 28725965.
- ↑ Kool, M.; Jones, DT.; Jäger, N.; Northcott, PA.; Pugh, TJ.; Hovestadt, V.; Piro, RM.; Esparza, LA. et al. (Mar 2014). "Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition.". Cancer Cell 25 (3): 393-405. doi:10.1016/j.ccr.2014.02.004. PMID 24651015.
- ↑ Gulino A, Arcella A, Giangaspero F (November 2008). "Pathological and molecular heterogeneity of medulloblastoma". Curr Opin Oncol 20 (6): 668–75. doi:10.1097/CCO.0b013e32831369f4. PMID 18841049.
- ↑ Rutkowski S, von Hoff K, Emser A, et al. (November 2010). "Survival and Prognostic Factors of Early Childhood Medulloblastoma: An International Meta-Analysis". J Clin Oncol 28 (33): 4961–4968. doi:10.1200/JCO.2010.30.2299. PMID 20940197.
- ↑ Rutkowski, S.; Bode, U.; Deinlein, F.; Ottensmeier, H.; Warmuth-Metz, M.; Soerensen, N.; Graf, N.; Emser, A. et al. (Mar 2005). "Treatment of early childhood medulloblastoma by postoperative chemotherapy alone.". N Engl J Med 352 (10): 978-86. doi:10.1056/NEJMoa042176. PMID 15758008.
- ↑ Ramaswamy, V.; Remke, M.; Bouffet, E.; Bailey, S.; Clifford, SC.; Doz, F.; Kool, M.; Dufour, C. et al. (Jun 2016). "Risk stratification of childhood medulloblastoma in the molecular era: the current consensus.". Acta Neuropathol 131 (6): 821-31. doi:10.1007/s00401-016-1569-6. PMID 27040285.