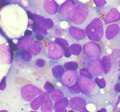
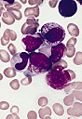
Leukemia
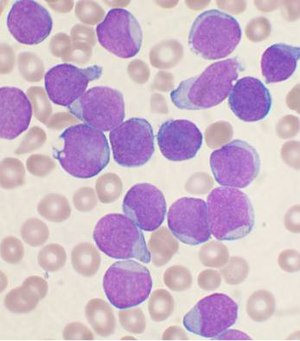
The article addresses leukemia, which is uncommonly seen by anatomical pathologists. It is a subset of hematopathology.
Lymphoma is discussed in the lymphoma article, and overlaps somewhat with leukemia as the clear distinction between the two is historical (see below).
Historical classification:[1]
- Leukemia = involves bone marrow +/- peripheral blood.
- Classic presentation: infection, bleeding, anemia.
- Lymphoma = discrete mass(es), usu. lymph node.
- Classic presentation: non-tender lymph nodes
Definition
All of the following:[2]
- Morphologic abnormalities.
- >20% blasts or recurrent cytogenetic abnormality.
Some recurrent cytogenetic abnormalities:
- t(8;21).
- inv(16).
- t(15;17).
Histomorphologic overview
| Disease/Feature | Blast size | Auer rods | Granulation of cytoplasm |
| Acute myeloid leukemia (AML) | larger | present | present |
| Acute lymphoid leukemia (ALL) | smaller † | none | absent or present |
† should be easy to remember as smALL people, i.e. kids, get this type of acute leukemia.
Algorithms
There is a nice set of algorithms from D. Arber - that were presented at the 2009 USCAP.
Clinical factors in classification
Clinical are important in the classification of leukemia.
- Hx of myelodysplastic syndrome?
- Chemotherapy?
- Down syndrome?
Leukemia classification
Acute myeloid leukemia (AML):
- AML.
- AML with recurrent cytogenetic abnormalities.
- AML from MDS.
- AML in the setting of Down syndrome.
Acute lymphoid leukemia (ALL):
- B cell.
- B cell with recurrent cytogenetic abnormalities.
- T cell.
Chronic myeloid leukemia (CML).
Prognosis
Highly dependent on health care system and treatment available[3]
- 5-year overall survival in children ranges:
- lymphoid leukaemia: 52.4% (Colombia) to 91.6% (Germany)
- acute myleoid leukemia: 33.3% (Bulgaria) to 78.2% (Germany)
Specific diagnoses
Acute myeloid leukemia
- Abbreviated AML.
General
- Adults.
Exclusions for this diagnosis:
- Prior MDS.
- Down syndrome.
Microscopic
Features:
- Auer rods present
- Cytoplasmic granularity.
- Large cells.
Note:
- May be classified by morphology, using the (old) French-American-British (FAB) classification (M0-M7).
Image
www:
Molecular
- Must exclude all the recurrent cytogenetic abnormalities - see below.
AML with recurrent cytogenetic abnormalities
Acute myeloid leukemia with t(8;21)
- t(8;21)(q22;q22).[4]
IHC:
- CD34+, CD13+, MPO+ (cytoplasm), CD33+ (weak).
- CD56+, CD117+.
- Usu. assoc. with a bad prognosis.
Flow cytometry:
- CD19+, PAX5+, CD79a +/-.
Images:
Acute myeloid leukemia with inv(16)
- inv(16)(p13.1q22).[5]
- Associated with myeloid sarcoma.
Microscopic:
- Blast count usu. ~20% (low).
- Eosinophilic granules.
- Used to be classified as "M4" with eosinophilia.
IHC:
- CD2+ -- common.
Acute myeloid leukemia with t(15;17)
- AKA acute promyelocytic leukemia
- Abbreviated APL.
- t(15;17)(q22;q12).
General
Clinical:
- Associated with DIC.
- Treatment: all-trans retinoic acid (ATRA).
Variants:
- t(11;17) -- ATRA doesn't work.[8]
- t(17;17) -- ATRA doesn't work.
- t(5;17). (???)
Microscopic
Comes in two flavours.
Microscopic (Hypergranular or typical APL):
- Bean-shaped nucleus or bilobed nucleus.
- Buddles of Auer rods - known as "Faggot cells".
Microscopic (Microgranular or hypogranular APL):
- Bilobed nuclei with nuclear overlap. (???)
- Absence of granules on light microscopy.
Images
www:
IHC
- CD2 +ve, CD34 +ve/-ve, CD56 +ve/-ve.
Flow cytometry
- CD34 -ve, HLA-DR -ve.
- CD33 +ve, CD13 +ve/-ve, CD117 +ve (weak), CD56 +ve/-ve.
Acute myeloid leukemia with t(9;11)
- t(9;11).
Microscopic:
- Monoblastic morphology. (???)
- Myelomonocytic morphology. (???)
Clinical:
- +/-DIC.
- Usu. children.
IHC:
- CD33+, CD65+, CD4+, HLA-DR+.
- CD34+. (???)
- CD13+. (???)
Chronic myeloid leukemia
Chronic myeloid leukaemia, abbreviated CML, is a type of myeloproliferative neoplasm. It is also known as chronic myelogenous leukemia.
It is driven by the bcr-abl fusion protein, detectable cytogenetically as the Philadelphia chromosome. It is a leukemia in that the fusion protein drives granulopoiesis (and less commonly megakaryopoiesis), resulting in marked peripheral blood leucocytosis.
General
- Adults - usually 50s or 60s.
Clinical - commonly:[10]
- Leukocytosis - neutrophils, myelocytes, metamyelocytes, +/-eosinophilia, +/-basophilia.
Progression:
- Chronic phase - potentially curable.
- Accelerated phase.
- Blast crisis.
Treatment:
- Imatinib and other tyrosine kinase inhibitors
Notes:
- Myeloblast (common granulocyte precursor) -> promyelocyte -> metamyelocyte -> myelocyte -> band -> mature myelocyte.[11]
Microscopic
Features:
- Bone marrow with too many granulocytes/granulocyte precursors.
- Granulocyte precursors:[11]
- Myeloblast (common granulocyte precursor) ~ 90% nucleus, multiple nucleoli.
- Should be less than 10%.
- Promyelocyte (committed to a specific linage (neutrophil, basophil or eosinophil)) - dia. 2x mature, 40-50% nucleus, one nucleolus.
- Metamyelocyte - dia. 2x mature, 30-40% nucleus, no nucleolus.
- Myelocyte - dia. 1x mature, 50-60% nucleus - kidney bean shape, no nucleolus.
- Band - dia. 1x mature, 30-40% nucleus - C shape/irregular, no nucleolus.
- Myeloblast (common granulocyte precursor) ~ 90% nucleus, multiple nucleoli.
- Granulocyte precursors:[11]
IHC
Rarely required. CML may transform into a blast phase, typically myeloid blasts, but lymphoblasts may also be seen. These blasts are typically CD34 and/or CD117 positive. The myeloid blasts will express myeloid markers and the lymphoblasts will shows lymphoid markers.
Molecular
- t(9;22) BCR-ABL - required for diagnosis.
- May be found in other leukemias.
See also
References
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 314. ISBN 978-1416054542.
- ↑ D. Good. 21 March 2011.
- ↑ Bonaventure, A.; Harewood, R.; Stiller, CA.; Gatta, G.; Clavel, J.; Stefan, DC.; Carreira, H.; Spika, D. et al. (Apr 2017). "Worldwide comparison of survival from childhood leukaemia for 1995-2009, by subtype, age, and sex (CONCORD-2): a population-based study of individual data for 89 828 children from 198 registries in 53 countries.". Lancet Haematol. doi:10.1016/S2352-3026(17)30052-2. PMID 28411119.
- ↑ Berger, R. (1994). "Translocation t(8;21)(q22;q22): cytogenetics and molecular biology.". Nouv Rev Fr Hematol 36 Suppl 1: S67-9. PMID 8177719.
- ↑ Lu, CM.; Murata-Collins, JL.; Wang, E.; Siddiqi, I.; Lawrence, HJ. (Dec 2006). "Concurrent acute myeloid leukemia with inv(16)(p13.1q22) and chronic lymphocytic leukemia: molecular evidence of two separate diseases.". Am J Hematol 81 (12): 963-8. doi:10.1002/ajh.20716. PMID 16917916.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 102578
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 180240
- ↑ Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 623 Q2. ISBN 978-1416025887.
- ↑ URL: http://path.upmc.edu/cases/case457.html. Accessed on: 21 January 2012.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 336. ISBN 978-1416054542.
- ↑ 11.0 11.1 URL: http://commons.wikimedia.org/wiki/File:Hematopoiesis_%28human%29_diagram.png. Accessed on: 14 January 2012.