Langerhans cell histiocytosis
Jump to navigation
Jump to search
The printable version is no longer supported and may have rendering errors. Please update your browser bookmarks and please use the default browser print function instead.

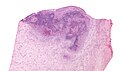
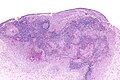
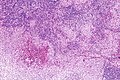
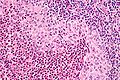
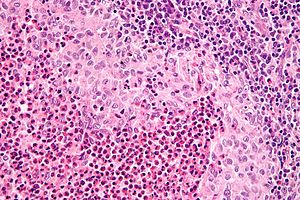
Langerhans cell histiocytosis. H&E stain. (WC/Nephron)
Langerhans cell histiocytosis, abbreviated LCH, is a rare disorder of tissue macrophages. It broadly fits into the category of histiocytoses. It used to known as eosinophilic granuloma.
It has been referred to by several eponyms - Hand-Schüller-Christian disease, Abt-Letterer-Siwe disease or Letterer-Siwe disease, and histiocytosis X.
This article deals with LCH in general. A separate article exists for pulmonary Langerhans cell histiocytosis.
General
Overview
LCH is really four (or three) diseases (depending on how one classifies it) - that happen to share the same histology:[1][2]
| Disease | Other name(s) | Prognosis | Demographic | Location | Risks/cause |
|---|---|---|---|---|---|
| Pulmonary Langerhans cell histiocytosis | Eosinophilic granuloma | good with smoking cessation | adults - smokers | lung only; typically upper lung field | due to smoking |
| Multifocal multisystem Langerhans cell histiocytosis | multisystem LCH, Letterer-Siwe disease | outcome dependent on organ involved,[3] natural history 2 year survival, 50% five year survival with treatment | usu. children < 2 years old, rarely adults[4] | multiple systems (skin, spleen, liver, lung, bone marrow) | possibly genetic ‡ |
| Unifocal Langerhans cell histiocytosis † | Eosinophilic granuloma | may spontaneously regress, may cure with surgery | children (?) | bone only | possibly genetic ‡ |
| Multifocal unisystem Langerhans cell histiocytosis † | multifocal LCH, eosinophilic granuloma, Hand-Schuller-Christian syndrome = bone defect, diabetes insipidus & exopthalmos | may spontaneously regress, may cure with surgery (?) | children (?) | usu. bone; may be in: skin, lungs, stomach | possibly genetic ‡ |
Note:
- † Robbins lumps these groups together.
- ‡ Incompletely understood. Somatic BRAF mutations identified in approximately half of the individuals.[5][6]
Clinical presentation
Features - dependent on subtype:[1]
- May present with fever, anemia, bone pain, bone fracture, diabetes insipidus, exophthalmos.
- Can be an incidental finding.
Microscopic
Features:[2]
- Langerhans cells histiocytes - key feature.
- Clusters of cells (histiocytes) with a reniform (kidney-shaped) nucleus and abundant foamy cytoplasm.
- Nucleus may look like a "coffee bean", i.e. have nuclear grooves (similar to those in papillary thyroid carcinoma) -- appearance dependent on the rotation of the nucleus.[7] May be called "buttock cells".
- Chromatin pattern: fine granular, light gray.
- Clusters of cells (histiocytes) with a reniform (kidney-shaped) nucleus and abundant foamy cytoplasm.
- +/-Eosinophils - often prominent.
- +/-Fibrosis - common.
- +/-Other inflammatory cells - neutrophils, plasma cells (uncommon).
- +/-Multinucleated giant cells - uncommon.
DDx:
- Kimura disease - eosinophilia.
- See lymph node pathology.
- See lesions with many eosinophils.
Images
www
IHC
Molecular
- Commonly have BRAF mutations ~ 40-70% of cases.[9]
- The V600E mutation is the most common BRAF mutation.[10]
- MAP2K1 mutations are often found in the cases without BRAF mutations.[9][11]
Electron microscopy
Etiology:
- Cell membrane invagination.[12]
Appearance:
- Electron dense, cytoplasmic tennis racket-like body.
Images:
See also
References
- ↑ 1.0 1.1 Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 338-9. ISBN 978-1416054542.
- ↑ 2.0 2.1 Chhabra, UD.; Desai, SS.; Jambhekar, NA. (Jul 2004). "Langerhans' cell histiocytosis: a clinicopathological study of 50 cases.". Indian J Pathol Microbiol 47 (3): 370-6. PMID 16295427.
- ↑ Minkov, M. (Apr 2011). "Multisystem Langerhans cell histiocytosis in children: current treatment and future directions.". Paediatr Drugs 13 (2): 75-86. doi:10.2165/11538540-000000000-00000. PMID 21351807.
- ↑ Garg, A.; Kumar, P. (Jan 2012). "Multisystem Langerhans cell histiocytosis in adult.". Indian J Dermatol 57 (1): 58-60. doi:10.4103/0019-5154.92683. PMID 22470214.
- ↑ Badalian-Very, G.; Vergilio, JA.; Degar, BA.; MacConaill, LE.; Brandner, B.; Calicchio, ML.; Kuo, FC.; Ligon, AH. et al. (Sep 2010). "Recurrent BRAF mutations in Langerhans cell histiocytosis.". Blood 116 (11): 1919-23. doi:10.1182/blood-2010-04-279083. PMID 20519626.
- ↑ Badalian-Very, G.; Vergilio, JA.; Degar, BA.; Rodriguez-Galindo, C.; Rollins, BJ. (Jan 2012). "Recent advances in the understanding of Langerhans cell histiocytosis.". Br J Haematol 156 (2): 163-72. doi:10.1111/j.1365-2141.2011.08915.x. PMID 22017623.
- ↑ BN. 15 March 2011.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 604862
- ↑ 9.0 9.1 Alayed, K.; Medeiros, LJ.; Patel, KP.; Zuo, Z.; Li, S.; Verma, S.; Galbincea, J.; Cason, RC. et al. (Feb 2016). "BRAF and MAP2K1 mutations in Langerhans cell histiocytosis: a study of 50 cases.". Hum Pathol. doi:10.1016/j.humpath.2015.12.029. PMID 26980021.
- ↑ Tatsuno, M.; Shioda, Y.; Iwafuchi, H.; Yamazaki, S.; Iijima, K.; Takahashi, C.; Ono, H.; Uchida, K. et al. (2016). "BRAF V600 mutations in Langerhans cell histiocytosis with a simple and unique assay.". Diagn Pathol 11 (1): 39. doi:10.1186/s13000-016-0489-z. PMID 27094161.
- ↑ Chakraborty, R.; Hampton, OA.; Shen, X.; Simko, SJ.; Shih, A.; Abhyankar, H.; Lim, KP.; Covington, KR. et al. (Nov 2014). "Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis.". Blood 124 (19): 3007-15. doi:10.1182/blood-2014-05-577825. PMID 25202140.
- ↑ URL: http://path.upmc.edu/cases/case147/micro.html. Accessed on: 7 January 2012.
- ↑ URL: http://path.upmc.edu/cases/case298.html. Accessed on: 14 January 2012.