Hodgkin lymphoma
| Classical Hodgkin lymphoma | |
|---|---|
| Diagnosis in short | |
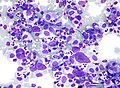
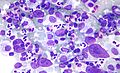
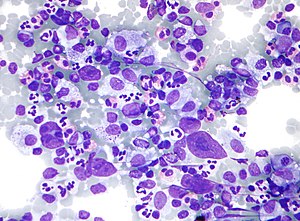 HL mixed cellularity - cytology. | |
|
| |
| LM | Reed-Sternberg cell (large binucleated cell (>= 45 micrometres), +/-multinucleated, +/-horseshoe-like shape, macronucleolus - approximately the size of a RBC (~8 micrometers)), well-defined cell border, abundant cytoplasm. |
| Subtypes | nodular sclerosis CHL, mixed cellularity CHL, lymphocyte-rich CHL, lymphocyte-depleted CHL |
| LM DDx |
diffuse large B cell lymphoma (esp. T-cell/histiocytic-rich LBCL), anaplastic large cell lymphoma, B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma, nodular lymphocyte-predominant Hodgkin lymphoma |
| IHC | CD30 Reed-Sternberg cells (RSCs) +ve ~98%, CD15 Reed-Sternberg cells +ve ~80% (also stains neutrophils), CD45 often negative in RSCs, CD20 -ve/+ve, PAX5 +ve |
| Site | usu. lymph node - classically in the neck |
|
| |
| Clinical history | young adults, older adults (bimodal distribution) |
| Signs | lymphadenopathy - usu. neck, +/-B symptoms (fever, night sweats, weight loss) |
| Prevalence | common |
| Prognosis | usu. good, dependent on stage |
| Nodular lymphocyte-predominant Hodgkin lymphoma | |
|---|---|
| Diagnosis in short | |
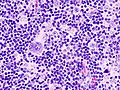
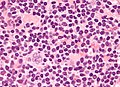
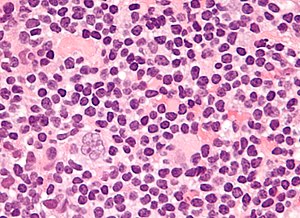 Popcorn cell in nodular lymphocyte-predominant Hodgkin lymphoma | |
|
| |
| LM | Popcorn cells (relatively) small (compared to classic RSCs) - have lobulated nucleus (key feature), small nucleoli; subtle nodularity at low power |
| Subtypes | none |
| LM DDx |
diffuse large B cell lymphoma (esp. T-cell/histiocytic-rich LBCL), anaplastic large cell lymphoma, B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma, classical Hodgkin lymphoma |
| IHC | LCA (CD45) +ve, CD20 +ve, CD10 +ve, Bcl-6 +ve, EMA +ve, CD30 -ve, CD15 -ve |
| Site | usu. lymph node |
|
| |
| Signs | lymphadenopathy |
| Prevalence | uncommon |
| Prognosis | good |
Hodgkin lymphoma, abbreviated HL, is a malignancy that often afflicts people in the prime of their life. Fortunately, it usually has a good prognosis.
Pathologists say "... it is both the easiest and hardest diagnosis to make." The reason for this is: the diagnosis depends on finding Reed-Sternberg cells (or Popcorn cells); if they are obvious the diagnosis is easy... if you can't find 'em and an alternative diagnosis is not apparent -- you wonder whether you're missing them.
General
Clinical
Symptoms:[1]
- "B symptoms" - all required:[2] weight loss, night sweats and fever.
- Infections due to immune dysfunction.
Diagnosis:
- HL cannot be diagnosed with standard flow cytometry (FC) - but has been diagnosed with specialized FC.[3]
Prognosis:
- Usually good.
- Unlike non-Hodgkin lymphomas, it spreads in a predictable pattern; thus, staging plays an important role in determining the therapy.[4]
Hodgkin lymphoma subtypes
Types:[1]
- Classical HL (CHL) - ~95% of HL.
- Nodular lymphocyte-predominant HL (NLPHL) - ~5% of HL.
Classic HL
- Four types of classic HL (see below).
NLPHL
- AKA lympho-histiocytic variant.
- Abbreviated NLPHL.
- Different IHC and morphologic appearance than classic HL.
- Significant risk for transformation into diffuse large B cell lymphoma (DLBCL); 10-year cumulative transformation rate (to DLBCL) in one study was 12%.[5]
Gross
Location:
- Almost always arises from a lymph node - classically in the neck.
- Extranodal Hodgkin lymphoma is (case report) rare.[6]
Microscopic
By definition, HL has Reed-Sternberg cells (RSCs) or Popcorn cells.
Classical HL
Features (classic HL):
- Reed-Sternberg cell.
- Large binucleated cell (>= 45 micrometres).[7]
- May be multinucleated.
- May have a horseshoe-like shape.
- Macronucleolus - approximately the size of a RBC (~8 micrometers).
- Well-defined cell border.
- Abundant cytoplasm.
- Large binucleated cell (>= 45 micrometres).[7]
Notes:
- Large mononuclear cells are common (so called "mononuclear RSCs") but not diagnostic.
Images (classic HL)
Subtypes of classic HL
There are four CHL subtypes:[1]
- Nodular sclerosis CHL - ~70% of CHL.
- Mixed cellular background - T cell, plasma cells, eosinophils, neutrophils and histiocytes.
- Nodular sclerosing fibrosis - thick strands fibrosis.
- Mixed cellularity CHL - ~20-25% of CHL.
- Lymphocyte-rich CHL - rare.
- T lymphocytes only (no mix of cells).
- Lymphocyte-depleted CHL - rare.
- May be associated with HIV infection.[8]
Memory device:
- The subtypes prevalence is in reverse alphabetical order.
Nodular lymphocyte predominant Hodgkin lymphoma
Features (nodular lymphocyte-predominant Hodgkin's lymphoma):
- Popcorn cell (previously known as Lymphocytic & histiocytic cell (L&H cell)[9]) - variant of RSC:
- Cells (relatively) small (compared to classic RSCs).
- Lobulated nucleus - key feature.
- Small nucleoli.
- Subtle nodularity at low power (2.5x or 5x objective).
Images (NLPHL)
www:
DDx both CHL & NLPHL
- CHL/NLPHL.
- Diffuse large B cell lymphoma (DLBCL), esp. T-cell/histiocytic-rich LBCL.
- Anaplastic large cell lymphoma (ALCL).
- B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma.[10]
IHC
Abbreviated panel:[11]
- CD30 Reed-Sternberg cells (RSCs) +ve ~98%
- CD15 Reed-Sternberg cells +ve ~80%, stains neutrophils.
- CD45 often negative in RSCs.
- CD20 may stain RSCs.
- PAX5 +ve.[12]
Additional - for completeness:
- CD3 (T lymphocytes)
NLPHL IHC differs from the classical HL:[12]
- LCA +ve.
- CD20 +ve.
- CD10 +ve.
- Bcl-6 +ve.
- EMA +ve.
- CD30 -ve
- CD15 -ve.
"UHN panel"
| Antibody | NLPHL | CHL |
| CD45 | +ve | -ve |
| CD20 | +ve | -ve |
| BCL6 | ||
| MUM1[13] | -ve | |
| CD30 | -ve | +ve (most sensitive). |
| CD15 | -ve | +ve |
| CD21 | networks present | no networks |
| CD23 | networks present | no networks |
| OCT-2 | +ve | -ve |
| PAX5 | +ve | +ve (proves B cell linage) |
| CD3 | usu. < benign B cell | usu. > benign B cell component |
| CD57 | rosettes around malign. cells | - |
| EBER | -ve | +ve/-ve |
| EMA | +ve/-ve | -ve |
| 4 unstained |
See also
References
- ↑ 1.0 1.1 1.2 Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 567. ISBN 978-0781765275.
- ↑ URL: http://lymphoma.about.com/od/symptoms/f/bsymptoms.htm. Accessed on: 11 August 2010.
- ↑ Fromm JR, Thomas A, Wood BL (March 2009). "Flow cytometry can diagnose classical hodgkin lymphoma in lymph nodes with high sensitivity and specificity". Am. J. Clin. Pathol. 131 (3): 322–32. doi:10.1309/AJCPW3UN9DYLDSPB. PMID 19228638.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 315. ISBN 978-1416054542.
- ↑ Biasoli I, Stamatoullas A, Meignin V, et al. (February 2010). "Nodular, lymphocyte-predominant Hodgkin lymphoma: a long-term study and analysis of transformation to diffuse large B-cell lymphoma in a cohort of 164 patients from the Adult Lymphoma Study Group". Cancer 116 (3): 631–9. doi:10.1002/cncr.24819. PMID 20029973.
- ↑ Vadmal, MS.; LaValle, GP.; DeYoung, BR.; Frankel, WL.; Marsh, WL. (Dec 2000). "Primary localized extranodal hodgkin disease of the transverse colon.". Arch Pathol Lab Med 124 (12): 1824-7. doi:10.1043/0003-9985(2000)1241824:PLEHDO2.0.CO;2. PMID 11100066.
- ↑ Mitchell, Richard; Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Aster, Jon (2011). Pocket Companion to Robbins & Cotran Pathologic Basis of Disease (8th ed.). Elsevier Saunders. pp. 329. ISBN 978-1416054542.
- ↑ 8.0 8.1 Sissolak G, Sissolak D, Jacobs P (April 2010). "Human immunodeficiency and Hodgkin lymphoma". Transfus. Apher. Sci. 42 (2): 131–9. doi:10.1016/j.transci.2010.01.008. PMID 20138008.
- ↑ Küppers R, Rajewsky K, Braeuninger A, Hansmann ML (March 1998). "L&H cells in lymphocyte-predominant Hodgkin's disease". N. Engl. J. Med. 338 (11): 763–4; author reply 764–5. doi:10.1056/NEJM199803123381113. PMID 9499174.
- ↑ Gualco, G.; Natkunam, Y.; Bacchi, CE. (Jan 2012). "The spectrum of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma: a description of 10 cases.". Mod Pathol. doi:10.1038/modpathol.2011.200. PMID 22222636.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 568. ISBN 978-0781765275.
- ↑ 12.0 12.1 Lefkowitch, Jay H. (2006). Anatomic Pathology Board Review (1st ed.). Saunders. pp. 683. ISBN 978-1416025887.
- ↑ URL: http://www.ncbi.nlm.nih.gov/omim/601900. Accessed on: 10 August 2010.