Colorectal tumours
Colorectal tumours, especially colorectal carcinomas, are very common. They are the bread and butter of GI pathology. Non-tumour colon is dealt with in the colon article.
An introduction to gastrointestinal pathology is in the gastrointestinal pathology article. The precursor lesion of colorectal carcinoma (CRC) is, typically, an adenomatous polyp. Polyps are discussed in the intestinal polyps article.
Classification
Most common
- Colon & rectum - most common = adenocarinoma.[1]
Others
Other tumours - many (incomplete list):[2]
- Mucinous carcinoma.
- Need > 50% mucinous component.[3]
- Adenosquamous carcinoma.
- Signet-ring carcinoma.
- Squamous carcinoma.
- Neuroendocrine neoplasms (carcinoid tumours).
- Lipoma.
- Leiomyoma.
- Gastrointestinal stromal tumour (GIST) - dealt with in a separate article.
- Angiosarcoma.
- Lymphoma (Non-Hodgkin's lymphoma).
Notes:
- Mucinous carcinoma - percentage required to call varies by site:
Squamous carcinoma
- Rare.
- In the context of a rectal tumour, retrograde growth from the anus should be considered.
Grading
- "Adenocarcinoma in situ" and "high-grade dysplasia" is used interchangeably by many in the colon and rectum.
- Splitting hairs - adenocarcinoma in situ is invasion into the lamina propria, high-grade dysplasia does not have lamina propria invasion. Ergo, the difference (in my opinion) amounts to seeing a desmoplastic stroma (adenocarcinoma) or not seeing one (dysplasia).
Grading of tumours:
- Tis - in situ (intramucosal).
- T1 - into submucosa (through mucularis mucosae).
- This is different than elsewhere, e.g. in the small bowel tumour cells in the lamina propria is defined as T1. The rationale for the T1 definition in CRC is that no lymphatics are present in the mucosa, ergo no risk of distant spread.
- T2 - into muscularis propria.
- T3 - into fat beyond musclaris propria.
- T4 - into something else.
Nodes:
- N0 - no positive nodes.
- N1 - 1-3 positive nodes.
- N2 - 4+ positive nodes.
Notes:
- Memory device for N2 = 4+ +ve LNs: the number four in chinese sounds like death.[4]
Staging of colorectal cancer
Simple version
Tumour/node grade for stage:[5]
- Stage I - T1 or T2 N0 M0.
- Stage II - T3 or T4 N0 M0.
- Stage III - Tx N1 or N2 M0.
- Stage IV - Tx Nx M1.
Complex version
Detailed tumour/node grade for stage:[6]
- Stage I - T1 or T2.
- Stage IIA - T3.
- Stage IIB - T4.
- Stage IIIA - T1 N1 or T2 N1.
- Stage IIIB - T3 N1 or T4 N1.
- Stage IIIC - Tx N2.
- Stage IV - Tx Nx M1.
Pathogenesis of colorectal carcinoma
Overview
Colorectal carcinoma is thought to arise from one of two pathways:[7][8]
- APC (adenomatous polyposis coli) gene mutation pathway, AKA classic adenoma-carcinoma pathway.
- Serrated pathway, AKA mutator pathway, mismatch repair pathway.
Syndromes
Both of the above described pathways are associated with syndromes:
- Familial adenomatous polyposis (FAP) or familial polyposis coli (FPC).
- Lynch syndrome (AKA hereditary non-polyposis colorectal cancer syndrome (HNPCC)).
Pathways
APC gene mutation pathway
Microscopic:
Mismatch repair pathway
- Associated with microsatellite instability (MSI).
- Common associated gene mutations:
- MLH1.
- PMS2.
- MSH2.
- MSH6.
- Less common gene mutations:
- PMS1.
- MLH3.
- MSH3.
IHC interpretation
- Loss of nuclear staining in nuclei of the tumour = mutation.
- Nuclear staining = normal.
MSI staining loss patterns:[9]
- MLH1 and PMS2 are often lost together, as MLH1 loss results in PMS2 loss.
- MSH2 and MSH6 are often lost together, as MSH2 loss results in MSH6 loss.
Implication of MSI staining loss patterns:
- PMS2 & MSH6 can be used as a screen.[9]
Etiology/significance loss of staining
- MSH2 mutations (IHC stain -ve) - often associated with a germline mutation,[10] while mutations in MLH1 are usually sporatic.[11]
- PMS2 mutations (IHC stain -ve) - often associated with a germline mutation.[12]
How to remember the more important MSI stuff:
- The MSHs are paired together.
- MSH (Mount Sinai Hospital) is where they started it in the city.
- PMS sucks... it's with the other one (MLH).
- The higher numbers in the pairings (PMS2, MSH6) are the screening tests (High Screen Pass).
- The 2s (MSH2, PMS2) are associated with germline mutations (Four legs good two legs bad!).
Other ancillary studies
- BRAF V600E missense mutation found in ~10% CRC.[13]
- KRAS mutation status.
BRAF V600E mutation
Features:[13]
- Independently associated with BRAF V600E:
- Usually older (>70 years old).
- Female gender.
- Right-sided tumour location.
- Worse prognosis - in the context of metastatic disease.
KRAS mutation
- Patient must have wild type KRAS to get drugs; KRAS mutation predicts resistance to cetuximab (Erbitux) and panitumumab (Vectibix).
- Cetuximab and panitumumab are EGFR inhibitors.
MSI cancers
General
Features:[16]
- Prognosis: slightly better than other CRC without MSI.
- Treatment implication: different response to chemotherapy.
MSI classification
MSI associated cancers can be classified into:[17][18]
- MSI-H >= 30% of loci have abnormality.
- MSI-L <30% of loci have abnormality.
Note:
- In the context of no chemotherapy, individuals with MSI-H tumours have a superior outcome to those with MSI-L tumours.[19]
- With chemotherapy the outcomes are similar.
Gross
Features:[16]
- Location: proximal colon, i.e. right-sided, predominance.
Microscopic
Features:[16]
- Lymphocytic infiltrate - see intratumoural lymphocytic response.
- Pushing border.[20]
- Histomorphology:
- Poorly differentiated.
- Mucinous.
- Signet ring.
- Medullary.[21]
Specific entities
Colorectal adenocarcinoma
General
- Very common.
- Rectum and sigmoid > proximal large bowel.
Presentation:
- Bright red blood per rectum (BRBPR).
- Constipation.
- Symptoms of bowel obstruction - nausea, vomiting.
Pathogenesis - see pathogenesis of colorectal carcinoma.
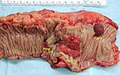
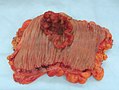
Gross
Often circumferential or near circumferential:
- These are referred to as "apple core lesion" or "napkin-ring" lesion.
Mucosa:
- Granular appearance.
- Raised (exophytic) or heaped edges with ulceration.
Note:
- Total mesorectal excisions should be assessed for completeness.
- The (soft tissue) radial margins, as present in TMEs and right hemicolectomies, should be inked.[22][23]
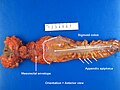
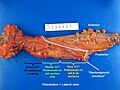
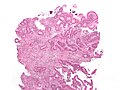
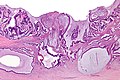
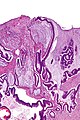
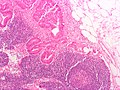
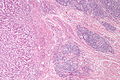
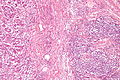
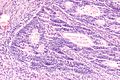
Images
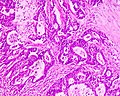
Microscopic
Features:
- Nuclear atypia:
- Nuclear pseudostratification.
- Nuclear hyperchromasia.
- Chromatin clearing or granularity.
- +/-Necrosis.
- Architecture - important for grading:
- Glands.
- Sheets.
Images
Mucinous adenocarcinoma - very low mag. (WC/Nephron)
CRC lymph node metastasis. (WC/Nephron)
www:
Grading
Based on component composed of glands:
- >=50% of tumour = low-grade (well-differentiated and moderately differentiated).
- <50% of tumour = high-grade (poorly-differentiated and undifferentiated).
Peritumour lymphocytic response
General
- Finding associated with improved survival in CRC.[26]
Microscopic
Onlinepathology advocates use of the Ueno criteria. They have a better inter-rater reproducibility than the older Graham criteria[27] and are less complicated.
Ueno criteria (2013)
Required criteria:[28]
- Non-MALT lymphoid aggregates (peritumoural) >= 1 mm.
Ignore:
- Muscosa-associated lymphoid tissue (MALT) = mucosal lymphoid aggregates, submucosal lymphoid aggregates adjacent to the musuclaris mucosae.
- Lymph nodes - these have a (fibrous) capsule.
- Irregular shape (not round).
Graham criteria (1990)
Required criteria:[27]
- Peritumoral:
- Lymphoid aggregates with germinal centres focally.
- Stellate fibrosis.
- No previous clinical and pathologic evidence of Crohn's disease.
Note:
- Should not be confused with intratumoural lymphocytic response.
- The intratumoural lymphocytic response is associated with MSI-H cancers.
Images
www:
- Peritumour lymphocytic response in endometrial carcinoma (bmjjournals.com).
- Peritumour lymphocytic response in CRC (ascpjournals.org).[29]
Intratumoural lymphocytic response
- AKA tumour-infiltrating lymphocytes, abbreviated TILs.
General
- Finding is suggestive of microsatellite instabillity.[30]
- May be seen in the context of Lynch syndrome.
Microscopic
Features:
- Lymphocytes are between the tumour cells.[31] †
- Other lymphocytes do not count.
Note:
- † Definitions vary substantially - some authors consider lymphocytes adjacent to the tumour (in the stroma around the tumour cells) "intratumoural".[32]
Images
www:
Tumour regression
There is a three tiered regression grading system by Ryan et al. for colorectal cancer that has essentially been adopted by CAP:[33]
| Grade | Features |
|---|---|
| Grade 1 | small groups of tumour cells or single tumour cells |
| Grade 2 | definite tumour but more fibrosis ("cancer outgrown by fibrosis") |
| Grade 3 | definite tumour with no fibrosis or tumour with a lesser amount of fibrosis ("fibrosis outgrown by cancer") |
IHC
- CK7 -ve.
- CK20 +ve.
- CEA +ve.
- CDX2 +ve.
Molecular
- KRAS mutation analysis.
- BRAF mutation analysis.
- V600E missense mutation found in ~10% CRC.[13]
Note:
- KRAS mutations and BRAF mutations are considered mutually exclusive as they occur in the same pathway.
See also
- Anus - covers anal cancer and anal intraepithelial neoplasia.
- Colon.
- Gastrointestinal pathology.
- Tumour budding.
References
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease (7th ed.). St. Louis, Mo: Elsevier Saunders. pp. 864. ISBN 0-7216-0187-1.
- ↑ Humphrey, Peter A; Dehner, Louis P; Pfeifer, John D (2008). The Washington Manual of Surgical Pathology (1st ed.). Lippincott Williams & Wilkins. pp. 198. ISBN 978-0781765275.
- ↑ Tozawa E, Ajioka Y, Watanabe H, et al. (2007). "Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: is mucinous carcinoma a distinct histological entity?". Pathol. Res. Pract. 203 (8): 567–74. doi:10.1016/j.prp.2007.04.013. PMID 17679024.
- ↑ URL: http://en.wikipedia.org/wiki/Numbers_in_Chinese_culture#Four. Accessed on: 28 June 2011.
- ↑ Shiau, Carolyn; Toren, Andrew (2006). Toronto Notes 2006: Comprehensive Medical Reference (Review for MCCQE 1 and USMLE Step 2) (22nd edition (2006) ed.). Toronto Notes for Medical Students, Inc.. pp. GS27. ISBN 978-0968592861.
- ↑ http://www.cancer.org/docroot/CRI/content/CRI_2_4_3X_How_is_colon_and_rectum_cancer_staged.asp
- ↑ Goldstein NS (January 2006). "Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification". Am. J. Clin. Pathol. 125 (1): 146–53. PMID 16483003.
- ↑ Rüschoff J, Aust D, Hartmann A (2007). "[Colorectal serrated adenoma: diagnostic criteria and clinical implications]" (in German). Verh Dtsch Ges Pathol 91: 119–25. PMID 18314605.
- ↑ 9.0 9.1 Hall, G.; Clarkson, A.; Shi, A.; Langford, E.; Leung, H.; Eckstein, RP.; Gill, AJ. (2010). "Immunohistochemistry for PMS2 and MSH6 alone can replace a four antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma.". Pathology 42 (5): 409-13. doi:10.3109/00313025.2010.493871. PMID 20632815.
- ↑ Mangold E, Pagenstecher C, Friedl W, et al. (December 2005). "Tumours from MSH2 mutation carriers show loss of MSH2 expression but many tumours from MLH1 mutation carriers exhibit weak positive MLH1 staining". J. Pathol. 207 (4): 385–95. doi:10.1002/path.1858. PMID 16216036.
- ↑ A. Pollett. 2010.
- ↑ Vaughn CP, Robles J, Swensen JJ, et al. (May 2010). "Clinical analysis of PMS2: mutation detection and avoidance of pseudogenes". Hum. Mutat. 31 (5): 588–93. doi:10.1002/humu.21230. PMID 20205264.
- ↑ 13.0 13.1 13.2 Tie J, Gibbs P, Lipton L, et al. (July 2010). "Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation". Int J Cancer. doi:10.1002/ijc.25555. PMID 20635392.
- ↑ Dunn EF, Iida M, Myers RA, et al. (October 2010). "Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab". Oncogene. doi:10.1038/onc.2010.430. PMID 20956938.
- ↑ Di Nicolantonio F, Martini M, Molinari F, et al. (December 2008). "Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer". J. Clin. Oncol. 26 (35): 5705–12. doi:10.1200/JCO.2008.18.0786. PMID 19001320.
- ↑ 16.0 16.1 16.2 Boland CR, Goel A (June 2010). "Microsatellite instability in colorectal cancer". Gastroenterology 138 (6): 2073–2087.e3. doi:10.1053/j.gastro.2009.12.064. PMID 20420947.
- ↑ Lawes DA, Pearson T, Sengupta S, Boulos PB (August 2005). "The role of MLH1, MSH2 and MSH6 in the development of multiple colorectal cancers". Br. J. Cancer 93 (4): 472–7. doi:10.1038/sj.bjc.6602708. PMC 2361590. PMID 16106253. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2361590/.
- ↑ Guidoboni M, Gafà R, Viel A, et al. (July 2001). "Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis". Am. J. Pathol. 159 (1): 297–304. PMC 1850401. PMID 11438476. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1850401/.
- ↑ Ribic, CM.; Sargent, DJ.; Moore, MJ.; Thibodeau, SN.; French, AJ.; Goldberg, RM.; Hamilton, SR.; Laurent-Puig, P. et al. (Jul 2003). "Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer.". N Engl J Med 349 (3): 247-57. doi:10.1056/NEJMoa022289. PMID 12867608.
- ↑ Pollet, A. 18 October 2010.
- ↑ Truta B, Chen YY, Blanco AM, et al. (2008). "Tumor histology helps to identify Lynch syndrome among colorectal cancer patients". Fam. Cancer 7 (3): 267–74. doi:10.1007/s10689-008-9186-8. PMID 18283560.
- ↑ URL: http://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13954. Accessed on: 6 February 2013.
- ↑ Bateman, AC.; Carr, NJ.; Warren, BF. (Apr 2005). "The retroperitoneal surface in distal caecal and proximal ascending colon carcinoma: the Cinderella surgical margin?". J Clin Pathol 58 (4): 426-8. doi:10.1136/jcp.2004.019802. PMID 15790712.
- ↑ Ogino, S.; Nosho, K.; Irahara, N.; Meyerhardt, JA.; Baba, Y.; Shima, K.; Glickman, JN.; Ferrone, CR. et al. (Oct 2009). "Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype.". Clin Cancer Res 15 (20): 6412-20. doi:10.1158/1078-0432.CCR-09-1438. PMID 19825961.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/Colon_12protocol_3200.pdf. Accessed on: 14 September 2012.
- ↑ Harrison, JC.; Dean, PJ.; el-Zeky, F.; Vander Zwaag, R. (Jan 1995). "Impact of the Crohn's-like lymphoid reaction on staging of right-sided colon cancer: results of multivariate analysis.". Hum Pathol 26 (1): 31-8. PMID 7821914.
- ↑ 27.0 27.1 Graham, DM.; Appelman, HD. (May 1990). "Crohn's-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator.". Mod Pathol 3 (3): 332-5. PMID 2362940.
- ↑ Ueno, H.; Hashiguchi, Y.; Shimazaki, H.; Shinto, E.; Kajiwara, Y.; Nakanishi, K.; Kato, K.; Maekawa, K. et al. (Apr 2013). "Objective Criteria for Crohn-like Lymphoid Reaction in Colorectal Cancer.". Am J Clin Pathol 139 (4): 434-41. doi:10.1309/AJCPWHUEFTGBWKE4. PMID 23525613.
- ↑ 29.0 29.1 Ross, JS.; Torres-Mora, J.; Wagle, N.; Jennings, TA.; Jones, DM. (Sep 2010). "Biomarker-based prediction of response to therapy for colorectal cancer: current perspective.". Am J Clin Pathol 134 (3): 478-90. doi:10.1309/AJCP2Y8KTDPOAORH. PMID 20716806.
- ↑ Iacopetta, B.; Grieu, F.; Amanuel, B. (Dec 2010). "Microsatellite instability in colorectal cancer.". Asia Pac J Clin Oncol 6 (4): 260-9. doi:10.1111/j.1743-7563.2010.01335.x. PMID 21114775.
- ↑ 31.0 31.1 Garg, K.; Soslow, RA. (Aug 2009). "Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma.". J Clin Pathol 62 (8): 679-84. doi:10.1136/jcp.2009.064949. PMID 19638537.
- ↑ Ropponen, KM.; Eskelinen, MJ.; Lipponen, PK.; Alhava, E.; Kosma, VM. (Jul 1997). "Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer.". J Pathol 182 (3): 318-24. doi:10.1002/(SICI)1096-9896(199707)182:3318::AID-PATH8623.0.CO;2-6. PMID 9349235.
- ↑ Ryan, R.; Gibbons, D.; Hyland, JM.; Treanor, D.; White, A.; Mulcahy, HE.; O'Donoghue, DP.; Moriarty, M. et al. (Aug 2005). "Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer.". Histopathology 47 (2): 141-6. doi:10.1111/j.1365-2559.2005.02176.x. PMID 16045774.
- ↑ Monzon, FA.; Ogino, S.; Hammond, ME.; Halling, KC.; Bloom, KJ.; Nikiforova, MN. (Oct 2009). "The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer.". Arch Pathol Lab Med 133 (10): 1600-6. doi:10.1043/1543-2165-133.10.1600. PMID 19792050.