Difference between revisions of "Adrenocortical carcinoma"
| Line 53: | Line 53: | ||
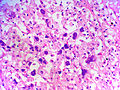
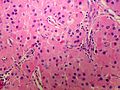
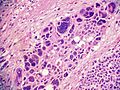
==Microscopic== | ==Microscopic== | ||
Various criteria exist for this diagnosis. The most widely used is the ''Weiss criteria'', which is a big long clunker. | Various criteria exist for this diagnosis. The most widely used is the ''Weiss criteria'', which is a big long clunker. | ||
In general: | |||
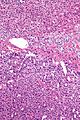
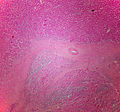
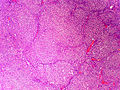
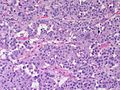
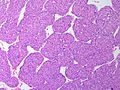
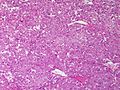
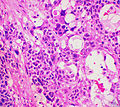
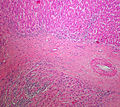
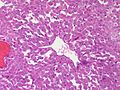
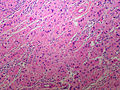
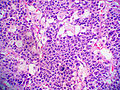
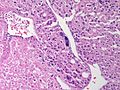
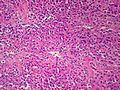
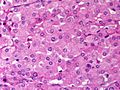
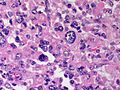
Adenomas are small, circumscribed and have cells with largely bland nuclei and abundant foamy clear or pink cytoplasm. Carcinomas are large, infiltrative, have fibrous bands and necrosis, and cells with less cytoplasm and more atypia. | |||
Notes: | Notes: | ||
Revision as of 11:17, 9 March 2015
| Adrenocortical carcinoma | |
|---|---|
| Diagnosis in short | |
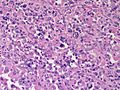
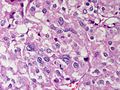
 Adrenocortical carcinoma. H&E stain. | |
|
| |
| Synonyms | adrenal cortical carcinoma |
|
| |
| LM | see below - various criteria, dependent on adult vs pediatric |
| IHC | vimentin +ve, melan A +ve, inhibin-alpha +ve, chromogranin A -ve, EMA -ve, S-100 -ve |
| Site | adrenal gland - cortex |
|
| |
| Syndromes | Li-Fraumeni syndrome, Beckwith-Wiedemann syndrome |
|
| |
| Prevalence | uncommon |
| Radiology | adrenal mass, typically large |
| Prognosis | poor |
| Clin. DDx | renal cell carcinoma, other abdominal masses |
| Treatment | surgical excision if feasible |
Adrenocortical carcinoma, abbreviated ACC, is a malignant tumour of the adrenal gland cortex.
It is also known as adrenal cortical carcinoma.
General
- A tumour of both children and adults.
- Prognosis poor, especially in adults.
Epidemiology:
- May be associated with a syndrome:[1]
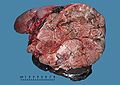
Gross
- +/-Encapsulated.
- Necrotic-appearing.
Image:
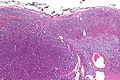
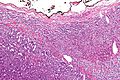
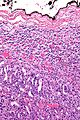
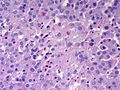
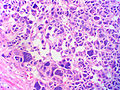
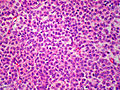
Microscopic
Various criteria exist for this diagnosis. The most widely used is the Weiss criteria, which is a big long clunker.
In general: Adenomas are small, circumscribed and have cells with largely bland nuclei and abundant foamy clear or pink cytoplasm. Carcinomas are large, infiltrative, have fibrous bands and necrosis, and cells with less cytoplasm and more atypia.
Notes:
- Tumour may contain fat.[2]
Images
www:
Adult
Weiss criteria
Three of the following:[3]
- High nuclear grade.
- High mitotic rate; >5/50 HPF (@ 40X obj.) - definition suffers from HPFitis.
- Atypical mitoses.
- Cleared cytoplasm in >= 25% of tumour cells.
- Sheeting (diffuse architecture) in >= 1/3 of tumour cells.
- Necrosis in nests.
- Venous invasion.
- Adrenal sinusoid invasion; lymphovascular space invasion within the adrenal gland.
- Capsular invasion.
Volante criteria
There is a simplified set of criteria by Volante et al. - that is not widely used:[4]
- Reticular network disruption (with reticulin staining).
- One of the three following:
- Abundant mitoses >5/50 high-power fields - definition suffers from HPFitis.
- Necrosis.
- Vascular invasion.
Pediatric
The criteria in the pediatric setting are somewhat different. This is discussed by Wieneke et al.[5] and Dehner and Hill.[6]
Dehner and Hill propose a very simple system:[6]
- "Low risk" < 200 g & confined to the adrenal.
- "Intermediate risk" 200-400 g, no mets, +/-microscopic disease outside adrenal.
- "High risk" >400 g, or mets, or gross invasion of adjacent structures.
IHC
- Vimentin +ve.
- Melan A +ve.
- Inhibin-alpha +ve.
- Cytokeratins +ve/-ve.
Others:
- Synaptophysin +ve/-ve.
- Chromogranin A -ve.
- Pheochromocytoma +ve.
- EMA -ve.
- Renal cell carcinoma +ve.
- S100 -ve.
- Pheochromocytoma +ve (sustentacular cells).[7]
- PAX-8 -ve.[8]
- CD10 +ve/-ve -- cannot be used to differentiate from RCC.[9]
See also
References
- ↑ Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; Aster, Jon (2009). Robbins and Cotran pathologic basis of disease (8th ed.). Elsevier Saunders. pp. 1157. ISBN 978-1416031215.
- ↑ Heye S, Woestenborghs H, Van Kerkhove F, Oyen R (2005). "Adrenocortical carcinoma with fat inclusion: case report". Abdom Imaging 30 (5): 641–3. doi:10.1007/s00261-004-0281-5. PMID 15688105.
- ↑ Jain M, Kapoor S, Mishra A, Gupta S, Agarwal A (2010). "Weiss criteria in large adrenocortical tumors: a validation study". Indian J Pathol Microbiol 53 (2): 222–6. doi:10.4103/0377-4929.64325. PMID 20551521.
- ↑ Volante M, Bollito E, Sperone P, et al. (November 2009). "Clinicopathological study of a series of 92 adrenocortical carcinomas: from a proposal of simplified diagnostic algorithm to prognostic stratification". Histopathology 55 (5): 535–43. doi:10.1111/j.1365-2559.2009.03423.x. PMID 19912359.
- ↑ Wieneke JA, Thompson LD, Heffess CS (July 2003). "Adrenal cortical neoplasms in the pediatric population: a clinicopathologic and immunophenotypic analysis of 83 patients". Am. J. Surg. Pathol. 27 (7): 867–81. PMID 12826878.
- ↑ 6.0 6.1 Dehner LP, Hill DA (2009). "Adrenal cortical neoplasms in children: why so many carcinomas and yet so many survivors?". Pediatr. Dev. Pathol. 12 (4): 284–91. doi:10.2350/08-06-0489.1. PMID 19326954.
- ↑ Unger P, Hoffman K, Pertsemlidis D, Thung S, Wolfe D, Kaneko M (May 1991). "S100 protein-positive sustentacular cells in malignant and locally aggressive adrenal pheochromocytomas". Arch. Pathol. Lab. Med. 115 (5): 484–7. PMID 1673596.
- ↑ Sangoi, AR.; Fujiwara, M.; West, RB.; Montgomery, KD.; Bonventre, JV.; Higgins, JP.; Rouse, RV.; Gokden, N. et al. (May 2011). "Immunohistochemical distinction of primary adrenal cortical lesions from metastatic clear cell renal cell carcinoma: a study of 248 cases.". Am J Surg Pathol 35 (5): 678-86. doi:10.1097/PAS.0b013e3182152629. PMID 21490444.
- ↑ Mete, O.; Kapran, Y.; Güllüoğlu, MG.; Kiliçaslan, I.; Erbil, Y.; Senyürek, YG.; Dizdaroğlu, F. (May 2010). "Anti-CD10 (56C6) is expressed variably in adrenocortical tumors and cannot be used to discriminate clear cell renal cell carcinomas.". Virchows Arch 456 (5): 515-21. doi:10.1007/s00428-010-0901-0. PMID 20390424.
.