Atypical small acinar proliferation
Atypical small acinar proliferation, abbreviated ASAP, is a small number of prostate glands that are abnormal and suspicious for carcinoma.
| Atypical small acinar proliferation | |
|---|---|
| Diagnosis in short | |
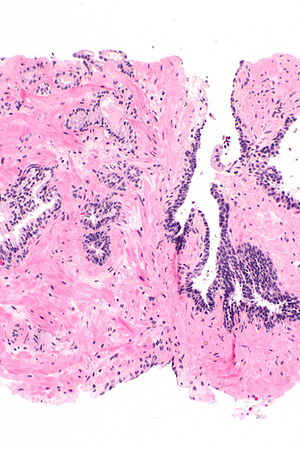 Atypical small acinar proliferation - top-left of image. H&E stain. | |
|
| |
| Synonyms | suspicious for prostate carcinoma |
|
| |
| LM | morphology of prostate carcinoma but less than 6 glands (major criteria for prostate carcinoma: abnormal architecture (increased gland density, usu. small circular glands, "infiltrative growth" pattern), basal cells lost, cytological abnormalities (nuclear enlargement, nucleoli); minor criteria for prostate carcinoma: nuclear hyperchromasia, wispy blue mucin, pink amorphous secretions, intraluminal crystalloid, amphophilic cytoplasm, adjacent HGPIN, mitoses) |
| LM DDx | prostate adenocarcinoma, benign prostate |
| IHC | AMACR +ve, CK34betaE12 -ve, p63 -ve, PSA +ve |
| Site | prostate gland |
|
| |
| Prevalence | ~3-5% of prostate biopsies |
| Blood work | +/-PSA elevated |
| Prognosis | increased risk of prostate carcinoma |
| Other | waffle diagnosis - used only on biopsy |
| Treatment | re-biopsy, close follow-up |
It is also known as suspicious for carcinoma.[1] ASAP is preferred as it does not contain the word carcinoma and, thus, cannot be misread as carcinoma, i.e. positive for malignancy.
General
- It is a waffle diagnosis, i.e. it is not considered an entity with a distinct pathobiology.[2]
- Analogous to ASCUS on a pap test.
- ASAP should be used sparingly.
- One benchmark is < 3-5% of biopsies.[1]
- Never diagnosed on excision, i.e. prostatectomy specimen.
- Cancers diagnosed in biopsies after ASAP are not more frequently clinically significant than cancers diagnosed after a diagnosis of benign or HGPIN.[3]
Association with adenocarcinoma
- On a subsequent biopsy the chance of finding adenocarcinoma is approximately 40%; this is higher than if there is high-grade prostatic intraepithelial neoplasia (HGPIN).[4]
Management
Microscopic
Features:
- Atypical appearing acini - see criteria for prostate adenocarcinoma.
- Limited extent - key feature.
- Less than six glands.†
Note:
- Deeper cuts didn't yield anything - important.
- † There is no agreed upon minimum number of glands; however, one paper suggests that agreement among experts is low with 5 or less glands.[7]
DDx:
- Prostatic adenocarcinoma.
- Benign prostate.
- Adenosis of the prostate.
- Sclerosing adenosis of the prostate.
Images
IHC
Usually stains like cancer:
- AMACR +ve.
- CK34betaE12 -ve.
- p63 -ve.
Note:
- Often not contributory.
Images
Sign out
C. Prostate, Left Base: - Atypical prostatic glands, suspicious for microfocus of adenocarcinoma.
Block letters
K. PROSTATE, LEFT LATERAL INTERIOR, BIOPSY: - ATYPICAL SMALL ACINAR PROLIFERATION.
See also
References
- ↑ 1.0 1.1 THvdK. 19 June 2010.
- ↑ Flury SC, Galgano MT, Mills SE, Smolkin ME, Theodorescu D (January 2007). "Atypical small acinar proliferation: biopsy artefact or distinct pathological entity". BJU International 99 (4): 780-5. PMID 17378841. http://www3.interscience.wiley.com/journal/118508438/abstract.
- ↑ Wiener, S.; Haddock, P.; Cusano, J.; Staff, I.; McLaughlin, T.; Wagner, J. (Dec 2017). "Incidence of Clinically Significant Prostate Cancer After a Diagnosis of Atypical Small Acinar Proliferation, High-grade Prostatic Intraepithelial Neoplasia, or Benign Tissue.". Urology 110: 161-165. doi:10.1016/j.urology.2017.08.040. PMID 28888752.
- ↑ Leite KR, Camara-Lopes LH, Cury J, Dall'oglio MF, Sañudo A, Srougi M (June 2008). "Prostate cancer detection at rebiopsy after an initial benign diagnosis: results using sextant extended prostate biopsy". Clinics 63 (3): 339–42. PMID 18568243. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322008000300009&lng=en&nrm=iso&tlng=en.
- ↑ Bostwick DG, Meiers I (July 2006). "Atypical small acinar proliferation in the prostate: clinical significance in 2006". Arch. Pathol. Lab. Med. 130 (7): 952–7. PMID 16831049. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=130&page=952.
- ↑ Rubin MA, Bismar TA, Curtis S, Montie JE (July 2004). "Prostate needle biopsy reporting: how are the surgical members of the Society of Urologic Oncology using pathology reports to guide treatment of prostate cancer patients?". Am. J. Surg. Pathol. 28 (7): 946–52. PMID 15223967.
- ↑ Van der Kwast, TH.; Evans, A.; Lockwood, G.; Tkachuk, D.; Bostwick, DG.; Epstein, JI.; Humphrey, PA.; Montironi, R. et al. (Feb 2010). "Variability in diagnostic opinion among pathologists for single small atypical foci in prostate biopsies.". Am J Surg Pathol 34 (2): 169-77. doi:10.1097/PAS.0b013e3181c7997b. PMID 20061936.