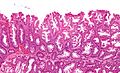
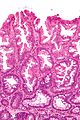
Sessile serrated adenoma
| Sessile serrated adenoma | |
|---|---|
| Diagnosis in short | |
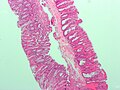
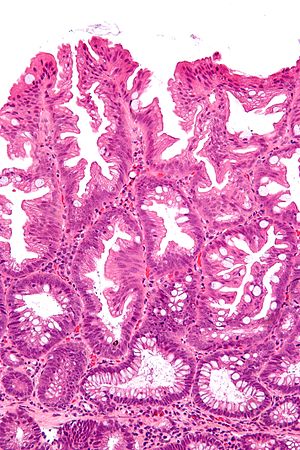 SSA. H&E stain. | |
|
| |
| Synonyms | sessile serrated lesion, sessile serrated polyp, sessile serrated adenoma/polyp |
|
| |
| LM | serrated epithelium, crypt base dilation, crypt branching, boot-shaped glands, horizontal glands |
| LM DDx | hyperplastic polyp, tubular adenoma when with dysplasia, mucosal prolapse for left sided lesions or background of diverticulosis |
| IHC | Chromogranin A (completely) -ve |
| Site | colon - usually cecum or ascending colon |
|
| |
| Associated Dx | colorectal adenocarcinoma, hyperplastic polyp |
| Syndromes | serrated polyposis syndrome, MUTYH polyposis syndrome |
|
| |
| Prevalence | common |
| Endoscopy | flat, usually > 5 mm, mucinous cap |
| Clin. DDx | normal, hyperplastic polyp, other intestinal polyps |
Sessile serrated adenoma, abbreviated SSA, is a premalignant polyp of the large bowel.
It is also known as sessile serrated polyp (abbreviated SSP), sessile serrated lesion and sessile serrated adenoma/polyp (abbreviated SSA/P). In the United Kingdom, this entity and is known as a sessile serrated lesion, a terminology that is likely to be adopted in the 2019/5th edition WHO Blue Book.
This lesion should not be confused with the traditional serrated adenoma, previously known as serrated adenoma.
General
- Colonic lesion.
- May be seen in the context of serrated polyposis syndrome.
- Approximately 5% of SSAs have dysplasia.[1]
Epidemiology:
- Thought to lead to colorectal cancer through a different pathway than most tumours in the left colon/rectum.
- Microvesicular hyperplastic polyps are hypothesized to be the the precursor of SSAs.[2]
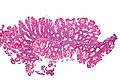
Gross
Features:[3]
- Flat lesions, usually > 5 mm.
- Typically have a "mucous cap" - present ~65% of the time; useful for identification.
- Border not well-demarcated.
- More common in the proximal colon.
Note:
- Sessile lesions over 1 cm are usually SSAs.[3]
Image:
Microscopic
Features:
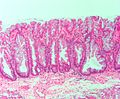
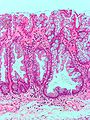
- Serrated epithelium at the surface and deep in the crypts.
- Saw-tooth appearance, epithelium has jagged appearing edge.
- Crypt dilation at base with serrations - key feature.
- Very common -- anecdotally the most sensitive feature.
- "Boot"-shape or "L"-shaped glands.
- Shape may be similar to a hockey stick.
- Horizontal crypts = crypt long axis parallel to the muscularis mucosae.
- Crypt branching.
- Submucosal lipoma or pseudolipoma is often seen in associated with SSA.[citation needed]
- Perineuriomas are also seen in a small proportion of cases
Minimal extent criteria - number of abnormal crypts with the above features:
- German Society of Pathology proposal: at least two abnormal crypts -- crypts do not have to be adjacent.[5][6]
- An expert panel lead by Rex states that one unequivocally altered crypt should prompt calling SSA.[3]
- The 4th edition of the WHO blue book requires - depending on what you read:
Dysplasia
Sessile serrated adenomas typically lack "conventional" nuclear atypia, as seen in adenomata in the tubulovillous spectrum. They are nonetheless neoplastic lesions on account of architectural "dysplasia". Additionally, dysplasia may manifest in more than one way:
- Intestinal or "cytological" dysplasia
- As seen in conventional adenomata, i.e. nuclear hyperchromasia and crowding. SSAs with nuclear atypia may be referred to as advanced sessile serrated adenomas
- Serrated dysplasia
- Round nuclei, prominent nucleoli and eosinophilic cytoplasm
- Minimal deviation dysplasia
- As the name suggests, there is only minor architectural and cytological changes. These areas are associated with loss of MLH1 immunostaining.[8]
DDx
- Hyperplastic polyp.
- Tubular adenoma - for SSA with dysplasia, TAs often less than 1 cm (uncommon for SSAs).
- Mucosal prolapse - especially for left sided lesions and a background of diverticulosis.[9]
Images
IHC
- Chromogranin A -ve; complete loss of staining.[10]
- Normal colorectal mucosa has scattered Chromogranin A-positive cells.
- Hyperplastic polyp has increased scattered Chromogranin A-positive cells.
Sign out
POLYP, CECUM, POLYPECTOMY: - SESSILE SERRATED ADENOMA. -- NEGATIVE FOR DYSPLASIA.
POLYP, ASCENDING COLON, POLYPECTOMY: - SESSILE SERRATED ADENOMA. -- NEGATIVE FOR DYSPLASIA.
POLYP, HEPATIC FLEXURE OF COLON, POLYPECTOMY: - SESSILE SERRATED ADENOMA. -- NEGATIVE FOR DYSPLASIA.
Dysplasia present
Polyp, Ascending Colon, Polypectomy or Biopsy:
- Sessile serrated adenoma with low-grade dysplasia, see comment.
Comment:
Sessile serrated adenomas with dysplasia are considered to be advanced lesions that
have an increased propensity to transform to adenocarcinoma. Complete endoscopic removal is recommended. If complete endoscopic removal cannot be achieved, short-term re-endoscopy and biopsy, or surgical resection should be considered.
Block letters
POLYP, ASCENDING COLON, POLYPECTOMY: - SESSILE SERRATED ADENOMA WITH DYSPLASIA.
The above mirrors the Canadian consensus.[11]
Sign out comment
The Canadian consensus[11] also advocates use of a comment, like the following statement:
Sessile serrated adenomas with dysplasia are considered to be advanced lesions that have an increased propensity to transform to adenocarcinoma. Complete endoscopic removal is recommended. If complete endoscopic removal cannot be achieved, short-term re-endoscopy and biopsy, or surgical resection should be considered.
Micro
The section shows a small polypoid fragment of colonic mucosa with a serrated epithelium that focally extends to the crypt base. Several dilated crypt bases are seen. One horizontal crypt and one boot-shaped crypt are present. The epithelium matures to the surface. A small amount of submucosa is present and contains a benign lymphoid aggregate.
See also
References
- ↑ Yang, JF.; Tang, SJ.; Lash, RH.; Wu, R.; Yang, Q. (Mar 2015). "Anatomic distribution of sessile serrated adenoma/polyp with and without cytologic dysplasia.". Arch Pathol Lab Med 139 (3): 388-93. doi:10.5858/arpa.2013-0523-OA. PMID 25724036.
- ↑ Huang, CS.; Farraye, FA.; Yang, S.; O'Brien, MJ. (Feb 2011). "The clinical significance of serrated polyps.". Am J Gastroenterol 106 (2): 229-40; quiz 241. doi:10.1038/ajg.2010.429. PMID 21045813.
- ↑ 3.0 3.1 3.2 Rex, DK.; Ahnen, DJ.; Baron, JA.; Batts, KP.; Burke, CA.; Burt, RW.; Goldblum, JR.; Guillem, JG. et al. (Sep 2012). "Serrated lesions of the colorectum: review and recommendations from an expert panel.". Am J Gastroenterol 107 (9): 1315-29; quiz 1314, 1330. doi:10.1038/ajg.2012.161. PMID 22710576.
- ↑ Rex DK, Hewett DG, Snover DC (December 2010). "Editorial: Detection targets for colonoscopy: from variable detection to validation". Am. J. Gastroenterol. 105 (12): 2665–9. doi:10.1038/ajg.2010.330. PMID 21131934.
- ↑ 5.0 5.1 Ensari, A.; Bilezikçi, B.; Carneiro, F.; Doğusoy, GB.; Driessen, A.; Dursun, A.; Flejou, JF.; Geboes, K. et al. (Nov 2012). "Serrated polyps of the colon: how reproducible is their classification?". Virchows Arch 461 (5): 495-504. doi:10.1007/s00428-012-1319-7. PMID 23052370.
- ↑ Aust, DE.; Baretton, GB. (Sep 2010). "Serrated polyps of the colon and rectum (hyperplastic polyps, sessile serrated adenomas, traditional serrated adenomas, and mixed polyps)-proposal for diagnostic criteria.". Virchows Arch 457 (3): 291-7. doi:10.1007/s00428-010-0945-1. PMID 20617338.
- ↑ URL: http://surgpathcriteria.stanford.edu/gitumors/sessile-serrated-polyp-adenoma/. Accessed on: 26 September 2012.
- ↑ Liu, C.; Walker, NI.; Leggett, BA.; Whitehall, VL.; Bettington, ML.; Rosty, C. (12 2017). "Sessile serrated adenomas with dysplasia: morphological patterns and correlations with MLH1 immunohistochemistry.". Mod Pathol 30 (12): 1728-1738. doi:10.1038/modpathol.2017.92. PMID 28752838.
- ↑ Huang, CC.; Frankel, WL.; Doukides, T.; Zhou, XP.; Zhao, W.; Yearsley, MM. (Apr 2013). "Prolapse-related changes are a confounding factor in misdiagnosis of sessile serrated adenomas in the rectum.". Hum Pathol 44 (4): 480-6. doi:10.1016/j.humpath.2012.06.011. PMID 23069257.
- ↑ Vitkovski T, Jawale R, Goldblum J et al. Density of neuroendocrine cells can distinguish hyperplastic polyps from small sessile serrated polyps. Modern Pathology (USCAP Annual Meeting 2018, Abstract Number 865), URL: https://www.nature.com/articles/modpathol20189.pdf. Accessed on: 10 May 2018.
- ↑ 11.0 11.1 Driman, DK.; Marcus, VA.; Hilsden, RJ; Owen, DA (2012). "Pathologic reporting of colorectal polyps: pan-Canadian consensus guidelines". Canadian Journal of Pathology 4 (3): 81-90. http://andrewjohnpublishing.com/images/cjp%204-3.pdf.