Difference between revisions of "Colorectal adenocarcinoma"
m |
(→General: wikify) |
||
| Line 42: | Line 42: | ||
Presentation: | Presentation: | ||
*Bright red blood per rectum (BRBPR). | *[[Bright red blood per rectum]] (BRBPR). | ||
*Constipation. | *Constipation. | ||
*Symptoms of bowel obstruction - nausea, vomiting. | *Symptoms of bowel obstruction - nausea, vomiting. | ||
Revision as of 03:49, 20 February 2014
| Colorectal adenocarcinoma | |
|---|---|
| Diagnosis in short | |
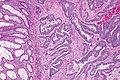
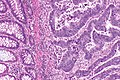
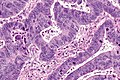
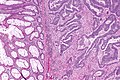
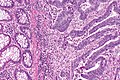
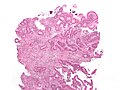
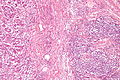
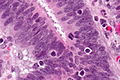
 Colorectal adenocarcinoma. H&E stain. | |
| LM DDx | other adenocarcinomas (e.g. anal gland adenocarcinoma, lung adenocarcinoma, ovarian adenocarcinoma), traditional adenoma esp. with high-grade dysplasia, sessile serrated adenoma with dysplasia |
| IHC | CK20 +ve, CDX2 +ve, CK7 -ve, beta-catenin (nuclear) +ve |
| Site | rectum, colon, cecum, appendix |
|
| |
| Associated Dx | long standing IBD (Crohn's disease, ulcerative colitis), traditional adenoma esp. with high-grade dysplasia, sessile serrated adenoma esp. with dysplasia |
| Syndromes | familial adenomatous polyposis, Lynch syndrome, Peutz-Jeghers syndrome, Juvenile polyposis syndrome, serrated polyposis syndrome, MUTYH polyposis syndrome, Cowden syndrome |
|
| |
| Signs | +/-blood in stools, +/-abdominal mass, +/-rectal mass, +/-signs of bowel obstruction (nausea, vomiting), +/-narrow caliber stools |
| Symptoms | +/-constipation |
| Prevalence | common |
| Blood work | +/-anemia (microcytic) |
| Radiology | +/-"apple core" lesion (classic), +/-findings of bowel obstruction (air-fluid levels esp. with transition point) |
| Endoscopy | +/-suspicious mass (exophytic or ulcerated), presence of non-lifting sign, Kudo pit pattern Type VI or Type VN |
| Prognosis | good to poor |
| Other | fecal occult blood test (FOBT) +ve |
| Clin. DDx | colorectal tumours, other causes - DDx dependent on presentation |
| Treatment | usually surgical resection +/-chemotherapy +/-radiation |
Colorectal adenocarcinoma is very common, and a leading cause of death due to cancer.
The colon and rectum are lumped together as the mucosa in the large bowel is very similar. Thus, colonic adenocarcinoma and rectal adenocarcinoma redirect to this article.
The larger generally topic of colorectal tumours and the pathogenesis of colorectal adenocarcinoma is dealt with in the colorectal tumours article.
Colorectal carcinoma, abbreviated CRC, is typically considered a synonym. Cecal adenocarcinoma is also lumped into CRC.
General
- Very common.
- Rectum and sigmoid > proximal large bowel.
Presentation:
- Bright red blood per rectum (BRBPR).
- Constipation.
- Symptoms of bowel obstruction - nausea, vomiting.
Pathogenesis - see pathogenesis of colorectal carcinoma.
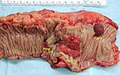
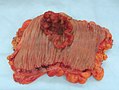
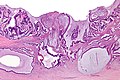
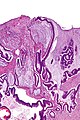
Gross
Often circumferential or near circumferential:
- These are referred to as "apple core lesion" or "napkin-ring" lesion.
Mucosa:
- Granular appearance.
- Raised (exophytic) or heaped edges with ulceration.
Note:
- Total mesorectal excisions should be assessed for completeness.
- The (soft tissue) radial margins, as present in TMEs and right hemicolectomies, should be inked.[1][2]
Images
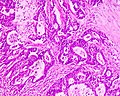
Microscopic
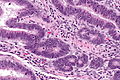
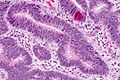
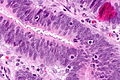
Features:
- Nuclear atypia:
- Nuclear pseudostratification.
- Nuclear hyperchromasia.
- Chromatin clearing or granularity.
- +/-Necrosis.
- Architecture - important for grading:
- Glands.
- Sheets.
Images
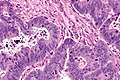
Mucinous adenocarcinoma - very low mag. (WC/Nephron)
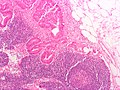
CRC lymph node metastasis. (WC/Nephron)
www:
Grading
Based on component composed of glands:
- >=50% of tumour = low-grade (well-differentiated and moderately differentiated).
- <50% of tumour = high-grade (poorly-differentiated and undifferentiated).
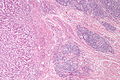
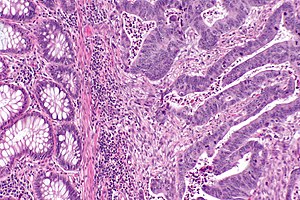
Peritumour lymphocytic response
General
- Finding associated with improved survival in CRC.[5]
Microscopic
Onlinepathology advocates use of the Ueno criteria. They have a better inter-rater reproducibility than the older Graham criteria[6] and are less complicated.
Ueno criteria (2013)
Required criteria:[7]
- Non-MALT lymphoid aggregates (peritumoural) >= 1 mm.
Ignore:
- Muscosa-associated lymphoid tissue (MALT) = mucosal lymphoid aggregates, submucosal lymphoid aggregates adjacent to the musuclaris mucosae.
- Lymph nodes - these have a (fibrous) capsule.
- Irregular shape (not round).
Graham criteria (1990)
Required criteria:[6]
- Peritumoral:
- Lymphoid aggregates with germinal centres focally.
- Stellate fibrosis.
- No previous clinical and pathologic evidence of Crohn's disease.
Note:
- Should not be confused with intratumoural lymphocytic response.
- The intratumoural lymphocytic response is associated with MSI-H cancers.
Images
www:
- Peritumour lymphocytic response in endometrial carcinoma (bmjjournals.com).
- Peritumour lymphocytic response in CRC (ascpjournals.org).[8]
Intratumoural lymphocytic response
- AKA tumour-infiltrating lymphocytes, abbreviated TILs.
General
- Finding is suggestive of microsatellite instabillity.[9]
- May be seen in the context of Lynch syndrome.
Microscopic
Features:
- Lymphocytes are between the tumour cells.[10] †
- Other lymphocytes do not count.
Note:
- † Definitions vary substantially - some authors consider lymphocytes adjacent to the tumour (in the stroma around the tumour cells) "intratumoural".[11]
Images
www:
Tumour deposits
General
Definition - TNM/AJCC 7th edition:[12]
- Microscopic or macroscopic nodule in the lymphatic drainage bed of the tumour.
- No standardized distance (from tumour) criteria are defined.[13]
- No findings suggestive of it being a lymph node replaced by tumour:
- No significant lymphoid tissue.
- Irregular contour.
- Round nodules of tumour are considered lymph nodes that are replaced by tumour.
Significance:
- Poor prognosticator.
- Can be understood as a type of invasive front/border, e.g. well-circumscribed border versus infiltrative border.[13]
Staging implications:
- Tumour deposits are not counted as (positive) lymph nodes[12] and in the context of positive lymph nodes do not change the N stage.
- If no positive lymph nodes are present, the N stage is pN1c.
- The T stage is not affected by tumour deposits.[12]
Note:
- The definition of tumour deposit has changed significantly between the TMN/AJCC fifth, sixth and seventh editions.[12]
Ueno criteria
Ueno et al. propose that a tumour deposit is either:[13]
- >=2 mm from the tumour front.
- >=2 mm (radially) from the deepest aspect of the muscularis propria, if the tumour is not present in the plane of section.
Tumour regression
There is a three tiered regression grading system by Ryan et al. for colorectal cancer that has essentially been adopted by CAP:[14]
| Grade | Features |
|---|---|
| Grade 1 | small groups of tumour cells or single tumour cells |
| Grade 2 | definite tumour but more fibrosis ("cancer outgrown by fibrosis") |
| Grade 3 | definite tumour with no fibrosis or tumour with a lesser amount of fibrosis ("fibrosis outgrown by cancer") |
IHC
- CK7 -ve.
- CK20 +ve.
- CEA +ve.
- CDX2 +ve.
Molecular
- KRAS mutation analysis.
- Mutation present ~ 40% of CRC.
- Mutations in codons 12 or 13 associated with failure of anti-EGFR therapy (e.g. cetuximab, panitumumab).[15]
- BRAF mutation analysis.
- V600E missense mutation found in ~10% CRC.[16]
Note:
- KRAS mutations and BRAF mutations are considered mutually exclusive as they occur in the same pathway.
Sign out
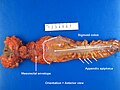
Right hemicolectomy
TERMINAL ILEUM, CECUM, ASCENDING COLON AND APPENDIX, RIGHT HEMICOLECTOMY: - INVASIVE ADENOCARCINOMA, LOW-GRADE, pT2, pN0. -- MARGINS NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY. -- PLEASE SEE TUMOUR SUMMARY. - SMALL BOWEL WALL WITHIN NORMAL LIMITS. - APPENDIX WITHOUT SIGNIFICANT PATHOLOGY. - TWELVE LYMPH NODES NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 12 ).
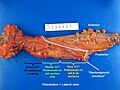
Mucinous component present
TERMINAL ILEUM, CECUM, ASCENDING COLON AND APPENDIX, RIGHT HEMICOLECTOMY: - INVASIVE ADENOCARCINOMA WITH A MUCINOUS COMPONENT, LOW-GRADE, pT1, pN0. -- MARGINS NEGATIVE FOR DYSPLASIA AND NEGATIVE FOR MALIGNANCY. -- PLEASE SEE TUMOUR SUMMARY. - SMALL BOWEL WALL WITHIN NORMAL LIMITS. - APPENDIX WITHOUT SIGNIFICANT PATHOLOGY. - FOURTEEN LYMPH NODES NEGATIVE FOR MALIGNANCY ( 0 POSITIVE / 14 ).
See also
References
- ↑ URL: http://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13954. Accessed on: 6 February 2013.
- ↑ Bateman, AC.; Carr, NJ.; Warren, BF. (Apr 2005). "The retroperitoneal surface in distal caecal and proximal ascending colon carcinoma: the Cinderella surgical margin?". J Clin Pathol 58 (4): 426-8. doi:10.1136/jcp.2004.019802. PMID 15790712.
- ↑ Ogino, S.; Nosho, K.; Irahara, N.; Meyerhardt, JA.; Baba, Y.; Shima, K.; Glickman, JN.; Ferrone, CR. et al. (Oct 2009). "Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype.". Clin Cancer Res 15 (20): 6412-20. doi:10.1158/1078-0432.CCR-09-1438. PMID 19825961.
- ↑ URL: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/Colon_12protocol_3200.pdf. Accessed on: 14 September 2012.
- ↑ Harrison, JC.; Dean, PJ.; el-Zeky, F.; Vander Zwaag, R. (Jan 1995). "Impact of the Crohn's-like lymphoid reaction on staging of right-sided colon cancer: results of multivariate analysis.". Hum Pathol 26 (1): 31-8. PMID 7821914.
- ↑ 6.0 6.1 Graham, DM.; Appelman, HD. (May 1990). "Crohn's-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator.". Mod Pathol 3 (3): 332-5. PMID 2362940.
- ↑ Ueno, H.; Hashiguchi, Y.; Shimazaki, H.; Shinto, E.; Kajiwara, Y.; Nakanishi, K.; Kato, K.; Maekawa, K. et al. (Apr 2013). "Objective Criteria for Crohn-like Lymphoid Reaction in Colorectal Cancer.". Am J Clin Pathol 139 (4): 434-41. doi:10.1309/AJCPWHUEFTGBWKE4. PMID 23525613.
- ↑ 8.0 8.1 Ross, JS.; Torres-Mora, J.; Wagle, N.; Jennings, TA.; Jones, DM. (Sep 2010). "Biomarker-based prediction of response to therapy for colorectal cancer: current perspective.". Am J Clin Pathol 134 (3): 478-90. doi:10.1309/AJCP2Y8KTDPOAORH. PMID 20716806.
- ↑ Iacopetta, B.; Grieu, F.; Amanuel, B. (Dec 2010). "Microsatellite instability in colorectal cancer.". Asia Pac J Clin Oncol 6 (4): 260-9. doi:10.1111/j.1743-7563.2010.01335.x. PMID 21114775.
- ↑ 10.0 10.1 Garg, K.; Soslow, RA. (Aug 2009). "Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma.". J Clin Pathol 62 (8): 679-84. doi:10.1136/jcp.2009.064949. PMID 19638537.
- ↑ Ropponen, KM.; Eskelinen, MJ.; Lipponen, PK.; Alhava, E.; Kosma, VM. (Jul 1997). "Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer.". J Pathol 182 (3): 318-24. doi:10.1002/(SICI)1096-9896(199707)182:3318::AID-PATH8623.0.CO;2-6. PMID 9349235.
- ↑ 12.0 12.1 12.2 12.3 Nagtegaal, ID.; Tot, T.; Jayne, DG.; McShane, P.; Nihlberg, A.; Marshall, HC.; Påhlman, L.; Brown, JM. et al. (Jun 2011). "Lymph nodes, tumor deposits, and TNM: are we getting better?". J Clin Oncol 29 (18): 2487-92. doi:10.1200/JCO.2011.34.6429. PMID 21555695.
- ↑ 13.0 13.1 13.2 Ueno, H.; Hashiguchi, Y.; Shimazaki, H.; Shinto, E.; Kajiwara, Y.; Nakanishi, K.; Kato, K.; Maekawa, K. et al. (Oct 2013). "Peritumoral deposits as an adverse prognostic indicator of colorectal cancer.". Am J Surg. doi:10.1016/j.amjsurg.2013.04.009. PMID 24112678.
- ↑ Ryan, R.; Gibbons, D.; Hyland, JM.; Treanor, D.; White, A.; Mulcahy, HE.; O'Donoghue, DP.; Moriarty, M. et al. (Aug 2005). "Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer.". Histopathology 47 (2): 141-6. doi:10.1111/j.1365-2559.2005.02176.x. PMID 16045774.
- ↑ Monzon, FA.; Ogino, S.; Hammond, ME.; Halling, KC.; Bloom, KJ.; Nikiforova, MN. (Oct 2009). "The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer.". Arch Pathol Lab Med 133 (10): 1600-6. doi:10.1043/1543-2165-133.10.1600. PMID 19792050.
- ↑ Tie J, Gibbs P, Lipton L, et al. (July 2010). "Optimizing targeted therapeutic development: Analysis of a colorectal cancer patient population with the BRAF(V600E) mutation". Int J Cancer. doi:10.1002/ijc.25555. PMID 20635392.