Difference between revisions of "Endometrial hyperplasia"
| Line 96: | Line 96: | ||
*[[Benign endometrial polyp]] - has thick-walled blood vessels; simple endometrial hyperplasia should not be diagnosed in a polyp.<ref name=pmid16873562/> | *[[Benign endometrial polyp]] - has thick-walled blood vessels; simple endometrial hyperplasia should not be diagnosed in a polyp.<ref name=pmid16873562/> | ||
Images | ====Images==== | ||
<gallery> | |||
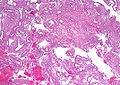
Image:Simple_endometrial_hyperplasia_-_low_mag.jpg | Simple endometrial hyperplasia - low mag. (WC) | |||
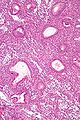
Image:Simple_endometrial_hyperplasia_-_high_mag.jpg | Simple endometrial hyperplasia - high mag. (WC) | |||
</gallery> | |||
==Simple endometrial hyperplasia with atypia== | ==Simple endometrial hyperplasia with atypia== | ||
Revision as of 03:37, 20 December 2013
- See Endometrium for an introduction to the topic.
Endometrial hyperplasia, abbreviated EH, is a precursor to endometrial carcinoma.
Overview
The most widely used system is from the World Health Organization (WHO).
WHO classification - overview
The WHO system is based on determining:
- Gland density (normal = simple hyperplasia, high density = complex hyperplasia).
- Presence/absence of nuclear atypia.
Alternate classifications - overview
Two alternative grading systems exist, that are (currently) not widely used:[1]
- European group of experts (1999).
- Endometrial collaborative group/Harvard (2000).
Both consist of two categories, as opposed to four found in the WHO classification.
European group of experts classification
- Endometrial hyperplasia.
- Endometrioid neoplasia.
Endometrial collaborative group/Harvard classification
- Endometrial hyperplasia.
- Endometrial intraepithelial neoplasia (EIN).
WHO classification
Management of endometrial hyperplasia
- Endometrial hyperplasia with atypia is usually treated with hysterectomy.[2]
- In women who want to maintain fertility it may be treated with progestin + short interval re-biopsies (q3 months).[3]
- Endometrial hyperplasia without atypia is treated by:
- Progestins + close follow-up OR hysterectomy.
Risk of progression to carcinoma
Approximate risk of progression to endometrial carcinoma - Latta rule of 3s:[4]
| Simple | Complex | |
| Without atypia | 1% | 3% |
| With atypia | 9% † | 27% ‡ |
Notes:
Ki-67
There is one paper that looks at Ki-67:[6]
| Diagnosis | Percent positive |
|---|---|
| Secretory phase endometrium | |
| Proliferative phase endometrium | |
| Simple hyperplasia | |
| Simple hyperplasia with atypia | |
| Complex hyperplasia | |
| Complex hyperplasia with atypia |
WHO system
Almost all hyperplasia is seen in the context of proliferative-type endometrium. Hyperplasia in the secretory-type endometrium is extremely rare and something diagnosed by or in consultation with an expert in gynecologic pathology.
Simple endometrial hyperplasia
- AKA simple hyperplasia.
General
- More common than simple endometrial hyperplasia with atypia.
- Very low risk for progressing to endometrioid endometrial carcinoma.
Microscopic
Features:[7]
- Irregular dilated glands (with large lumens) - key feature.
- Glands described as "animal shapes".
- Variation of gland size.
- No nuclear atypia.
- Uniform columnar nuclei.
- Normal gland density (gland area in plane of section/total area ~= 1/3).
DDx:
- Disordered proliferative phase.
- Complex endometrial hyperplasia - has increased gland-to-stroma ratio.
- Cystic atrophy of the endometrium - does not have proliferative activity.[8]
- Benign endometrial polyp - has thick-walled blood vessels; simple endometrial hyperplasia should not be diagnosed in a polyp.[8]
Images
Simple endometrial hyperplasia with atypia
General
- Very uncommon.
Microscopic
Features:[7]
- Irregular dilated glands (with large lumens) - important feature.
- Glands described as "animal shapes".
- Variation of gland size.
- No nuclear atypia.
- Uniform columnar nuclei.
- Normal gland density (gland area in plane of section/total area ~= 1/3).
- Nuclear atypia:[9]
- Loss of basal nuclear stratification.
- Nuclear size variation.
- Nuclear rounding.
- Nuclei lacking atypical = uniform columnar nuclei.
- Nucleoli.
- Hyperchromasia or vesicular nuclei.
Notes:
- There are no clear criteria for atypia. Different sources list different features.
- VL criteria for atypia (all should be present):
- Increased NC ratio.
- Atypical: ~ 1:2
- Not atypical: ~1:3.
- Oval nuclei with small major axis to minor axis ratio.
- Atypical: major axis:minor axis = <=2:1.
- Not atypical: major axis:minor axis = >=3:1
- NB: round nuclei: major axis:minor axis = 1:1.
- Small nucleoli (~1/5 the size of the nucleus).
- Increased NC ratio.
Complex endometrial hyperplasia
- Abbreviated CEH.
Complex endometrial hyperplasia with atypia
- AKA complex atypical hyperplasia.
Other
Endometrial hyperplasia with secretory changes
General
- Rare.
- Secretory changes seen in 1-2% of endometrial hyperplasias/endometrial carcinomas.[10]
Microscopic
Features:[11]
- Secretory changes - includes at least one of three following:[12]
- Stromal decidualization.
- Cytoplasmic vacuolization.
- Intraluminal secretions.
- Proliferative-type epithelium. †
- Mitoses.
- Nuclear atypia.
- Pseudostratified epithelium.
Notes:
- † This is not precisely defined. I suppose it is some of the things Bell and Ostrezega[13] mention (mitoses, nuclear atypia, pseudostratified epithelium).
- Bell and Ostrezega[13] give a laundry list for differentiating benign secretory endometrium from hyperplasia with secretory changes: focal architectural abnormalities, metaplastic ciliated & "clear" cells, sharp luminal border, epithelial pseudopalisading, nuclear atypia, vesicular nuclei, mitoses.
DDx:
Images:
See also
References
- ↑ Dietel, M. (Nov 2001). "The histological diagnosis of endometrial hyperplasia. Is there a need to simplify?". Virchows Arch 439 (5): 604-8. PMID 11764378.
- ↑ URL: http://www.aafp.org/afp/990600ap/3069.html.
- ↑ URL: http://www.aafp.org/afp/20060801/practice.html.
- ↑ Latta, E. January 2009.
- ↑ 5.0 5.1 Kurman, RJ.; Kaminski, PF.; Norris, HJ. (Jul 1985). "The behavior of endometrial hyperplasia. A long-term study of untreated hyperplasia in 170 patients.". Cancer 56 (2): 403-12. PMID 4005805.
- ↑ Abike, F.; Tapisiz, OL.; Zergeroglu, S.; Dunder, I.; Temizkan, O.; Temizkan, I.; Payasli, A. (2011). "PCNA and Ki-67 in endometrial hyperplasias and evaluation of the potential of malignancy.". Eur J Gynaecol Oncol 32 (1): 77-80. PMID 21446331.
- ↑ 7.0 7.1 Nucci, Marisa R.; Oliva, Esther (2009). Gynecologic Pathology: A Volume in Foundations in Diagnostic Pathology Series (1st ed.). Churchill Livingstone. pp. 236. ISBN 978-0443069208.
- ↑ 8.0 8.1 McCluggage, WG. (Aug 2006). "My approach to the interpretation of endometrial biopsies and curettings.". J Clin Pathol 59 (8): 801-12. doi:10.1136/jcp.2005.029702. PMC 1860448. PMID 16873562. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1860448/.
- ↑ Silverberg, SG. (Mar 2000). "Problems in the differential diagnosis of endometrial hyperplasia and carcinoma.". Mod Pathol 13 (3): 309-27. doi:10.1038/modpathol.3880053. PMID 10757341.
- ↑ Simon RA, Hansen K, Xiong JJ, et al. PTEN status and frequency of endometrial carcinoma and its precursors arising in functional secretory endometrium; an immunohistochemical study of 29 cases. Mod Pathol. 2012;25(Suppl 2): 1248A.
- ↑ Simon RA. CAP Today. June 2012. Accessed on: 24 April 2013.
- ↑ Tresserra, F.; Lopez-Yarto, M.; Grases, PJ.; Ubeda, A.; Pascual, MA.; Labastida, R. (Mar 2003). "Endometrial hyperplasia with secretory changes.". Gynecol Oncol 88 (3): 386-93. PMID 12648591.
- ↑ 13.0 13.1 Bell, CD.; Ostrezega, E. (Aug 1987). "The significance of secretory features and coincident hyperplastic changes in endometrial biopsy specimens.". Hum Pathol 18 (8): 830-8. PMID 3610133.